Abstract
Objectives
The addition of bevacizumab to standard chemotherapy has been improved survival outcomes in patients with metastatic colorectal cancer. However, the combination of bevacizumab with oxaliplatin-based chemotherapy as first-line treatment showed limited survival benefit. The purpose of this study was to investigate the clinical efficacy and toxicity of the combination of bevacizumab to oxaliplatin and leucovorin (FOLFOX4) in the first-line treatment of patient with metastatic colorectal cancer.
Methods
Between December 2004 and September 2009, medical records of patients who were diagnosed with metastatic colorectal cancer and received the first line chemotherapy with bevacizumab and FOLFOX4, were retrospectively reviewed.
Results
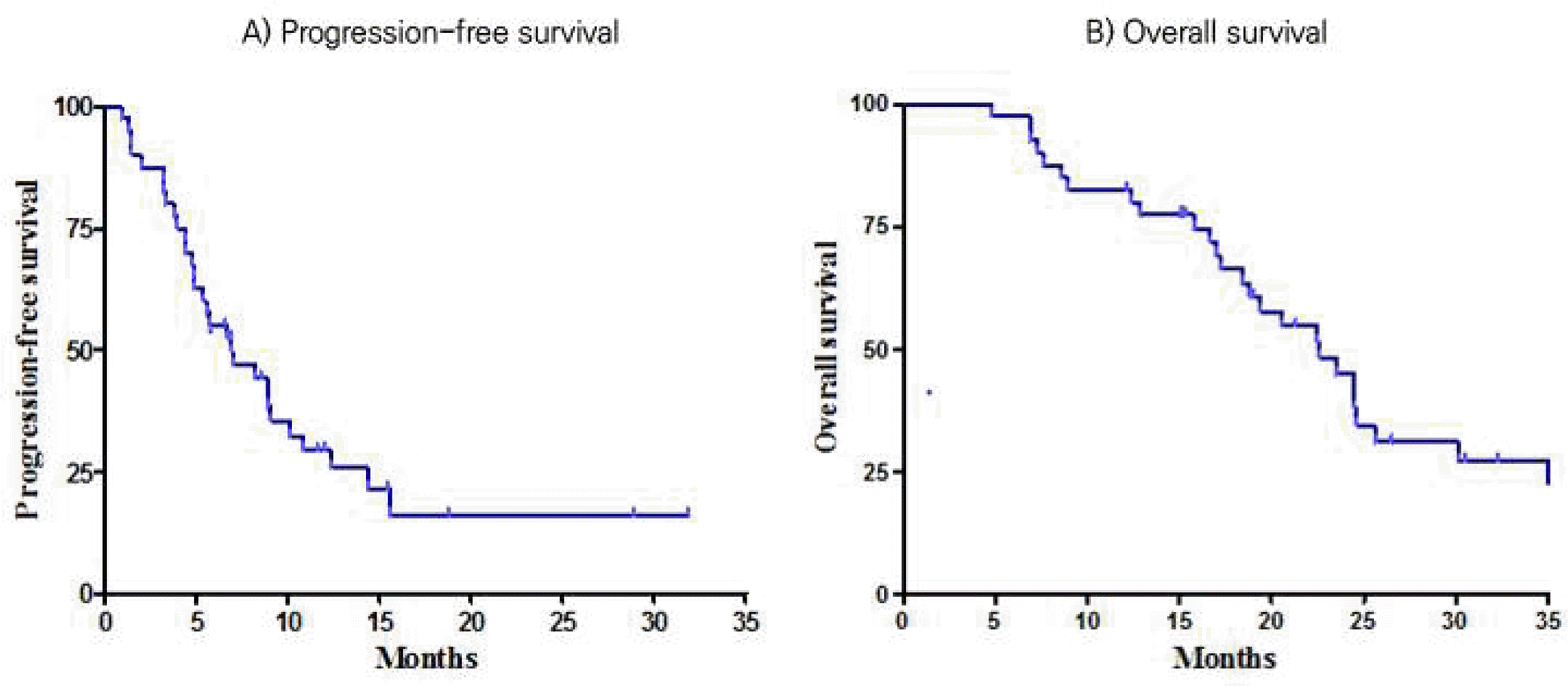
A total of forty patients were analyzed. The median age of the patients was 55 years (range, 33-80), and 55% was male. The patients received a total of 206 cycles of therapy (median 4 cycles per patient; range 1 – 15 cycles). Of these 40 patients, none achieved complete response (CR) and 15 achieved a partial response (PR), for the overall response rate (ORR) 37.5% (95% CI, 22.5-52.5). Median progression free survival (PFS) was 6.9 months (95% CI, 3.4-10.5) and median overall survival (OS) was 22.6 months (95% CI, 17.3-27.8The most common grade 3 or 4 hematologic toxicity and non-hematologic toxicity were neutropenia (10.0%) and diarrhea (10.0%), respectively. Two patients experienced gastrointestinal perforation.
REFERENCES
1.de Gramont A., Figer A., Seymour M., Homerin M., Hmissi A., Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000. 18:2938–47.

2.Douillard JY., Cunningham D., Roth AD., Navarro M., James RD., Karasek P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000. 355:1041–7.

3.Saltz LB., Cox JV., Blanke C., Rosen LS., Fehrenbacher L., Moore MJ, et al. Irinotecan plus Fluorouracil and Leucovorin for Metastatic Colorectal Cancer. Irinotecan Study Group. N Engl J Med. 2000. 343:905–14.
4.Goldberg RM., Sargent DJ., Morton RF., Fuchs CS., Ramanathan RK., Williamson SK, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004. 22:23–30.

5.Saltz LB., Clarke S., Diaz-Rubio E., Scheithauer W., Figer A., Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008. 26:2013–9.

6.Van Cutsem E., Köhne CH., Hitre E., Zaluski J., Chang Chien CR., Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009. 360:1408–17.

7.Douillard JY., Siena S., Cassidy J., Tabernero J., Burkes R., Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010. 28:4697–705.

8.Kabbinavar F., Hurwitz HI., Fehrenbacher L., Meropol NJ., Novotny WF., Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003. 21:60–5.

9.Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004. 350:2335–42.

10.Kabbinavar FF., Schulz J., McCleod M., Patel T., Hamm JT., Hecht JR, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005. 23:3697–705.

11.Macedo LT., da Costa Lima AB., Sasse AD. Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: a systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC cancer. 2012. 12:89.

12.Meyerhardt JA., Li L., Sanoff HK., Carpenter W 4th., Schrag D. Effectiveness of bevacizumab with first-line combination chemotherapy for Medicare patients with stage IV colorectal cancer. J Clin Oncol. 2012. 30:608–15.

13.Grothey A., Sargent D., Goldberg RM., Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004. 22:1209–14.

14.Petrelli F., Coinu A., Ghilardi M., Cabiddu M., Zaniboni A., Barni S. Efficacy of oxaliplatin-based chemotherapy + bevacizumab as first-line treatment for advanced colorectal cancer: a systematic review and pooled analysis of published trials. Am J Clin Oncol. 2015. 38:227–33.
15.Petrelli F., Borgonovo K., Cabiddu M., Ghilardi M., Lonati V., Maspero F, et al. FOLFIRI-bevacizumab as first-line chemotherapy in 3500 patients with advanced colorectal cancer: a pooled analysis of 29 published trials. Clin Colorectal Cancer. 2013. 12:145–51.

17.Gressett SM., Shah SR. Intricacies of bevacizumab-induced toxicities and their management. Ann Pharmacother. 2009. 43:490–501.

18.Ranpura V., Hapani S., Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA. 2011. 305:487–94.
19.Hapani S., Chu D., Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009. 10:559–68.

20.Heinzerling JH., Huerta S. Bowel perforation from bevacizumab for the treatment of metastatic colon cancer: incidence, etiology, and management. Curr Surg. 2006. 63:334–7.

Table 1.
Patient characteristics (n = 40)
Table 2.
Toxicity profile (According to NCI-CTCAE1)version3.0)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download