Abstract
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disorder of the central nervous system that leads to neurological disability. The diagnosis of MS relies on the MRI criteria, which can demonstrate dissemination in space and time. Exclusion of other demyelinating mimics is essential because there are no specific biomarker for MS and MRI criteria are still have imperfect. There is incremental improvements in MS treatment option that have contributed to the delay of disease progression. The early initiation of DMT may ameliorate the neurological disability. In this review, we discusses the new diagnostic MS criteria and summarize the evidences supporting the early treatment in the course of MS.
Go to : 
REFERENCES
2.Miller DH., Chard DT., Ciccarelli O. Clinically isolated syndromes. Lancet Neurol. 2012. 11:157–69.

3.Miller D., Barkhof F., Montalban X., Thompson A., Filippi M. Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurol. 2005. 4:281–8.

4.Miller DH., Weinshenker BG., Filippi M., Banwell BL., Cohen JA., Freedman MS, et al. Differential diagnosis of suspected multiple sclerosis: a consensus approach. Mult Scler. 2008. 14:1157–74.

5.McDonald WI., Compston A., Edan G., Goodkin D., Hartung HP., Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001. 50:121–7.

6.Polman CH., Reingold SC., Edan G., Filippi M., Hartung HP., Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005. 58:840–6.

7.Polman CH., Reingold SC., Banwell B., Clanet M., Cohen JA., Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011. 69:292–302.

8.Filippi M., Rocca MA., Ciccarelli O., De Stefano N., Evangelou N., Kappos L, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016. 15:292 –303.

9.Sombekke MH., Wattjes MP., Balk LJ., Nielson JM., Vrenken H., Uitdehaag BM, et al. Spinal cord lesions in patients with clinically isolated syndrome: a powerful tool in diagnosis and prognosis. Neurology. 2013. 80:69–75.

10.Weier K., Mazraeh J., Naegelin Y., Thoeni A., Hirsch JG., Fabbro T, et al. Biplanar MRI for the assessment of the spinal cord in multiple sclerosis. Mult Scler. 2012. 18:1560–9.

11.Okuda DT., Mowry EM., Beheshtian A., Waubant E., Baranzini SE., Goodin DS, et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology. 2009. 72:800–5.

12.Horowitz AL., Kaplan RD., Grewe G., White RT., Salberg LM. The ovoid lesion: a new MR observation in patients with multiple sclerosis. AJNR Am J Neuroradiol. 1989. 10:303–5.
13.Grossman RI., Barkhof F., Filippi M. Assessment of spinal cord damage in MS using MRI. J Neurol Sci. 2000. 15:172 Suppl. 1:S36–9.

14.Lucchinetti CF., Popescu BF., Bunyan RF., Moll NM., Roemer SF., Lassmann H, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011. 365:2188–97.

15.Calabrese M., De Stefano N., Atzori M., Bernardi V., Mattisi I., Barachino L, et al. Detection of cortical inflammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis. Arch Neurol. 2007. 64:1416–22.

16.Filippi M., Rocca MA., Calabrese M., Sormani MP., Rinaldi F., Perini P, et al. Intracortical lesions: relevance for new MRI diagnostic criteria for multiple sclerosis. Neurology. 2010. 75:1988–94.

17.Kilsdonk ID., Lopez-Soriano A., Kuijer JP., de Graaf WL., Castelijns JA., Polman CH, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014. 261:1356–64.
18.Tallantyre EC., Dixon JE., Donaldson I., Owens T., Morgan PS., Morris PG, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011. 76:534–9.

19.Charil A., Yousry TA., Rovaris M., Barkhof F., De Stefano N., Fazekas F, et al. MRI and the diagnosis of multiple sclerosis: expanding the concept of “no better explanation”. Lancet Neurol. 2006. 5:841–52.

20.Kim SH., Kim W., Li XF., Jung IJ., Kim HJ. Does interferon beta treatment exacerbate neuromyelitis optica spectrum disorder? Mult Scler. 2012. 18:1480–3.

21.Wingerchuk DM., Banwell B., Bennett JL., Cabre P., Carroll W., Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015. 85:177–89.

22.Weisfeld-Adams JD., Katz Sand IB., Honce JM., Lublin FD. Differential diagnosis of Mendelian and mitochondrial disorders in patients with suspected multiple sclerosis. Brain. 2015. 138:517–39.

23.Pfeffer G., Burke A., Yu-Wai-Man P., Compston DA., Chinnery PF. Clinical features of MS associated with Leber hereditary optic neuropathy mtDNA mutations. Neurology. 2013. 81:2073–81.

24.Rovaris M., Gambini A., Gallo A., Falini A., Ghezzi A., Benedetti B, et al. Axonal injury in early multiple sclerosis is irreversible and independent of the short-term disease evolution. Neurology. 2005. 65:1626–30.

25.Filippi M., Rovaris M., Inglese M., Barkhof F., De Stefano N., Smith S, et al. Interferon beta-1a for brain tissue loss in patients at presentation with syndromes suggestive of multiple sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet. 2004. 364:1489–96.

26.Baysal Kıraç L., Ekmekçi Ö., Yüceyar N., Sağduyu Kocaman A. Assessment of early cognitive impairment in patients with clinically isolated syndromes and multiple sclerosis. Behav Neurol. 2014. 2014:637694.

27.Filippi M., Horsfield MA., Morrissey SP., MacManus DG., Rudge P., McDonald WI, et al. Quantitative brain MRI lesion load predicts the course of clinically isolated syndromes suggestive of multiple sclerosis. Neurology. 1994. 44:635–41.

28.Jacobs LD., Beck RW., Simon JH., Kinkel RP., Brownscheidle CM., Murray TJ, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis CHAMPS Study Group. N Engl J Med. 2000. 343:898–904.
29.Kinkel RP., Dontchev M., Kollman C., Skaramagas TT., O'Connor PW., Simon JH. Association between immediate initiation of intramuscular interferon beta-1a at the time of a clinically isolated syndrome and long-term outcomes: a 10-year follow-up of the Controlled High-Risk Avonex Multiple Sclerosis Prevention Study in Ongoing Neurological Surveillance. Arch Neurol. 2012. 69:183–90.
30.Comi G., Filippi M., Barkhof F., Durelli L., Edan G., Fernández O, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet. 2001. 357:1576–82.

31.Comi G., Martinelli V., Rodegher M., Moiola L., Bajenaru O., Carra A, et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet. 2009. 374:1503–11.

32.Kappos L., Polman CH., Freedman MS., Edan G., Hartung HP., Miller DH, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006. 67:1242–9.

33.Kappos L., Edan G., Freedman MS., Montalbán X., Hartung HP., Hemmer B, et al. The 11-year long-term follow-up study from the randomized BENEFIT CIS trial. Neurology. 2016. 87:978–87.

34.Miller AE., Wolinsky JS., Kappos L., Comi G., Freedman MS., Olsson TP, et al. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014. 13:977–86.

35.Kavaliunas A., Manouchehrinia A., Stawiarz L., Ramanujam R., Agholme J., Hedström AK, et al. Importance of early treatment initiation in the clinical course of multiple sclerosis. Mult Scler. 2016.

36.Hohlfeld R. & Wekerle, H. Autoimmune concepts of multiple sclerosis as a basis for selective immunotherapy: from pipe dreams to (therapeutic) pipelines. Proc Natl Acad. Sci USA. 2004. 101(Suppl 2):14599–606.
37.Ransohoff RM., Hafler DA., Lucchinetti CF. Multiple sclerosis-a quiet revolution. Nat Rev Neurol. 2015. 11:134–42.
Go to : 
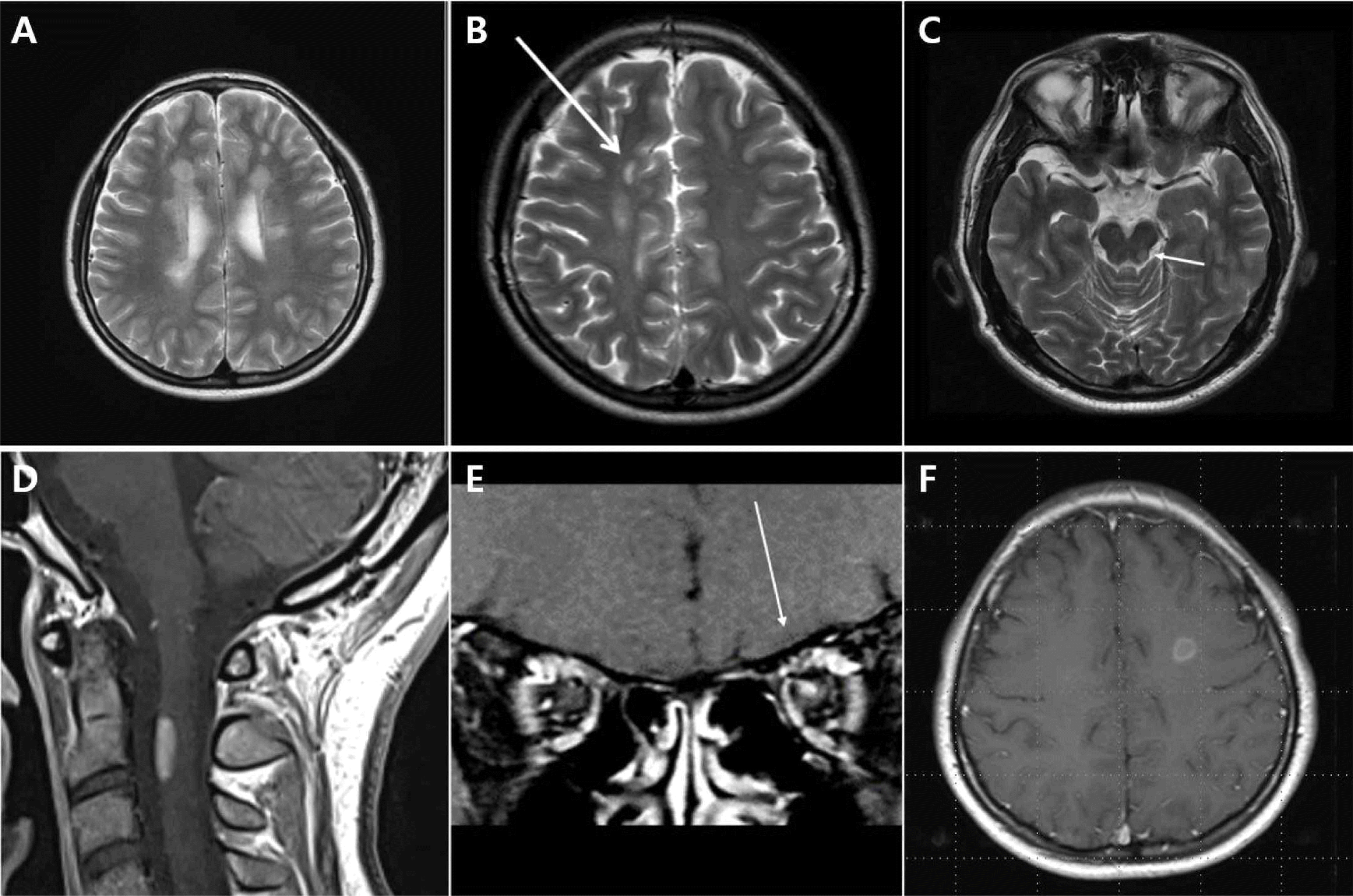 | Fig. 1.Example of lesion for multiple sclerosis MRI Criteria of dissemination in space (A) periventricular lesions; (B) juxtacortical lesions; (C) infratentorial lesions; (D) spinal cord lesion; (E) optic nerve lesion. Gadolinium-enhancing lesion in T1 gadolinium weighted image; (F) are visible in the patient with MS. |
Table 1.
CIS clinical features and likelihood of signaling an MS diagnosis4
Table 2.
2010 McDonald criteria and 2016 MAGNIMS Diagnosis criteria8
Table 3.
Clinical and MRI major red flags suggestive of alternative diagnosis to multiple sclerosis4




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download