Abstract
Objectives
This research was carried out to identify the synergistic effect of dexmedetomidine and ketorolac on neuropathic pain alleviation.
Methods
The anti-allodynic effect of intrathecal dexmedetomidine and ketorolac was investigated in rats after L5 spinal nerve ligation (SNL). Mechanical allodynia was assessed using Von Frey filaments. Every day for 3 consecutive days, beginning on the 10th day after SNL, behavioral tests were carried out at 1, 2, and 4 hr after drug injection.
Results
Significant increases in ipsilateral paw withdrawal thresholds (PWTs) were observed 1, 2, and 4 hr after drug injection in the groups of rats which received intrathecal injection of either dexmedetomidine (group D) or ketorolac (group K), compared to group S (P< 0.05). And group DK, which received simultaneous intrathecal injection of both dexmedetomidine and ketorolac, showed statistically significantly higher ipsilateral PWTs than groups D and K, which received only one of them (P< 0.05).
Conclusions
The results of this research demonstrated the anti-allodynic effect of dexmedetomidine and ketorolac on neuropathic pain induced by SNL in rats. They also suggest that synergistic analgesia can be induced by the simultaneous injection of dexmedetomidine and ketorolac, and that combination therapy is an effective approach to treating chronic neuropathic pain syndrome.
Go to : 
References
1. Jensen TS, Baron R, Haanpää M, Kalso E, Loeser JD, Rice AS, et al. A new definition of neuropathic pain. Pain. 2011; 152:2204–5.

2. D'Angelo R, Morreale A, Donadio V, Boriani S, Maraldi N, Plazzi G, et al. Neuropathic pain following spinal cord injury: what we know about mechanisms, assessment and management. Eur Rev Med Pharmacol Sci. 2013; 17:3257–61.
3. Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur J Pharmacol. 1988; 150:9–14.
4. Asano T, Dohi S, Ohta S, Shimonaka H, Iida H. Antinociception by epidural and systemic α (2)-adrenoceptor agonists and their binding affinity in rat spinal cord and brain. Anesth Analg. 2000; 90:400–7.
5. Kimura M, Saito S, Obata H. Dexmedetomidine decreases hyperalgesia in neuropathic pain by increasing acetylcholine in the spinal cord. Neurosci Lett. 2012; 529:70–4.

6. Zhang H, Zhou F, Li C, Kong M, Liu H, Zhang P, et al. Molecular mechanisms underlying the analgesic property of intrathecal dexmedetomidine and its neurotoxicity evaluation: an in vivo and in vitroexperimental study. PLoS One. 2013; 8:e55556.
7. Brocks DR, Jamali F. Clinical pharmacokinetics of ketorolac tromethamine. Clin Pharmacokinet. 1992; 23:4215–27.

8. Malmberg AB, Yaksh TL. Antinociceptive effects of intrathecal nonsteroidal antiinflammatory drugs (NSAIDs) on tonic pain behavior in rats. Anesthesiology. 1992; 77:A732.

9. Malmberg AB, Yaksh TL. Pharmacology of the spinal action of ketorolac, morphine, ST-91, U50488H, and L-PIA on the formalin test and an isobalographic analysis of the NSAID int-teraction. Anesthesiology. 1993; 79:270–81.
10. Guan Y, Johanek LM, Hartke TV, Shim B, Tao YX, Ringkamp M, et al. Peripherally acting mu-opioid receptor agonist attenuates neuropathic pain in rats after L5 spinal nerve injury. Pain. 2008; 138:318–29.

11. Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976; 17:1031–6.

12. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63.

13. Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980; 20:441–62.

14. Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidencebased recommendation. Pain. 2007; 132:237–51.
15. Hayashida K, Obata H, Nakajima K, Eisenach JC. Gabapentin acts within the locus coeruleus to alleviate neuropathic pain. Anesthesiology. 2008; 109:1077–84.

16. Hayashida K, Parker R, Eisenach JC. Oral gabapentin activates spinal cholinergic circuits to reduce hypersensitivity after peripheral nerve injury and interacts synergistically with oral donepezil. Anesthesiology. 2007; 106:1213–9.

18. Sonohata M, Furue H, Katafuchi T, Yasaka T, Doi A, Kumamoto E, et al. Actions of noradrenaline on substantia gelatinosa neurones in the rat spinal cord revealed by in vivo patch recording. J Physiol. 2004; 555:515–26.
19. Paqueron X, Conklin D, Eisenach JC. Plasticity in action of intrathecal clonidine to mechanical but not thermal nociception after peripheral nerve injury. Anesthesiology. 2003; 99:199–204.

20. Eisenach JC, DuPen S, Dubois M, Miguel R, Allin D. Epidural clonidine analgesia for intractable cancer pain. The epidural clonidine study group. Pain. 1995; 61:391–9.
21. Hayashida K, Eisenach JC. Spinal alpha 2-adrenoceptor-mediated analgesia in neuropathic pain reflects brain-derived nerve growth factor and changes in spinal cholinergic neuronal function. Anesthesiology. 2010; 113:406–12.
22. Ramwell PW, Shaw JE, Jessup R. Spontaneous and evoked release of prostaglandins from frog spinal cord. Am J Physiol. 1966; 211:998–1012.

23. Malmberg AB, Yaksh TL. Hyperalgesia mediated by spinal glutamate or substance P receptor blocked by spinal cyclooxygenase inhibition. Science. 1992; 257:1276–9.

24. Pellerin M, Hardy F, Abergel A. Chronic refractory pain in cancer patients: Value of the spinal injection of lysine acetylsalicylate. Presse Med. 1987; 16:1465–8.
25. Uda R, Horiguchi S, Ito S, Hyodo M, Hayaishi O. Nociceptive effects induced by intrathecal administration of prostaglandin D2, E2, or F2α to conscious mice. Brain Res. 1990; 510:26–32.

Go to : 
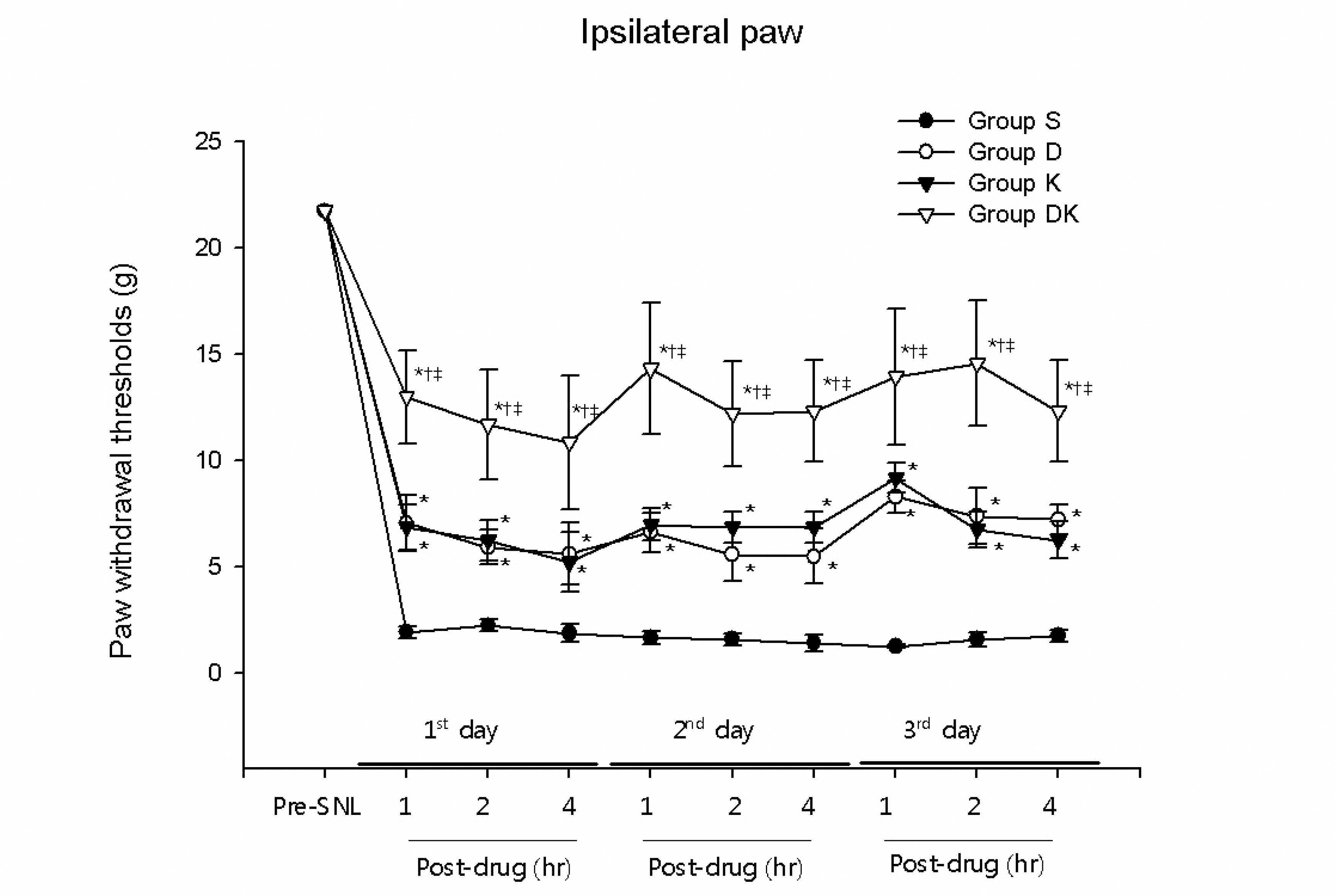 | Fig. 1.The alleviating effect of intrathecal dexmedetomidine and ketorolac on mechanical allodynia in spinal nerve ligation rats. All the rats underwent SNL. Group S (n = 5) received intrathecal injection of normal saline, while groups D (n = 5) and K (n = 5) received intrathecal injection of dexmedetomidine and ketorolac, respectively. Group DK (n = 5) received simultaneous intrathecal injection of both dexmedetomidine and ketorolac. At 1, 2, and 4 hr after drug injection, on 3 consecutive days beginning on the 10th day after SNL, statistically significant increases in ipsilateral paw withdrawal thresholds were shown by groups D and K compared to group S (P< 0.05); and by Group DK compared to groups D and K (P< 0.05). ∗P< 0.05 compared to Group S, † P< 0.05 compared to Group D. ‡P< 0.05 compared to Group K.
|
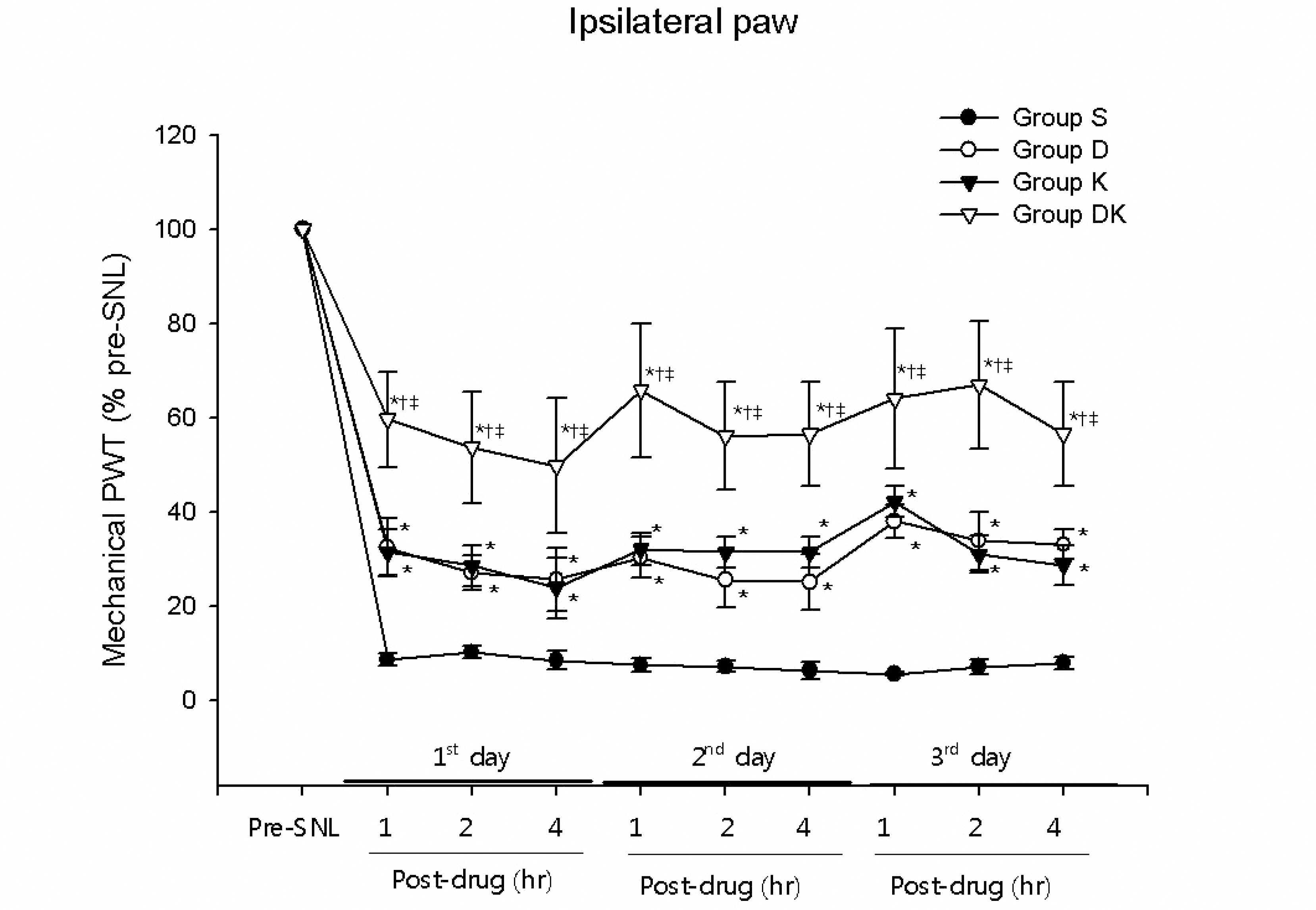 | Fig. 2.Increases in ipsilateral paw withdrawal thresholds in spinal nerve ligation rats caused by intrathecal dexmedetomidine and ketorola. Regarding % pre-SNL in ipsilateral paw, significantly higher PWTs were shown by groups D and K compared to group S at 1, 2, and 4hr after drug injection, on 3 consecutive days beginning on the 10th day after SNL (P< 0.05); and by group DK compared to groups D and K (P< 0.05). ∗P< 0.05 compared to Group S, †P< 0.05 compared to Group D. ‡P< 0.05 compared to Group K.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download