Abstract
Objective
The imbalance between pro-inflammatory and anti-inflammatory cytokines may underlie different pain states. Although ascorbic acid is the most important physiological antioxidant that affects host defense mechanisms and immune homeostasis, there is limited information on the effects of ascorbic acid on the production of cytokines.
Methods
In this study, we investigated the in vitro effect of L-ascorbic acid (AA) on the production of pro-inflammatory and anti-inflammatory cytokines by stimulating C57BL/6 mouse splenocytes with the polyclonal activators lipopolysaccharide or concanavalin A.
Results
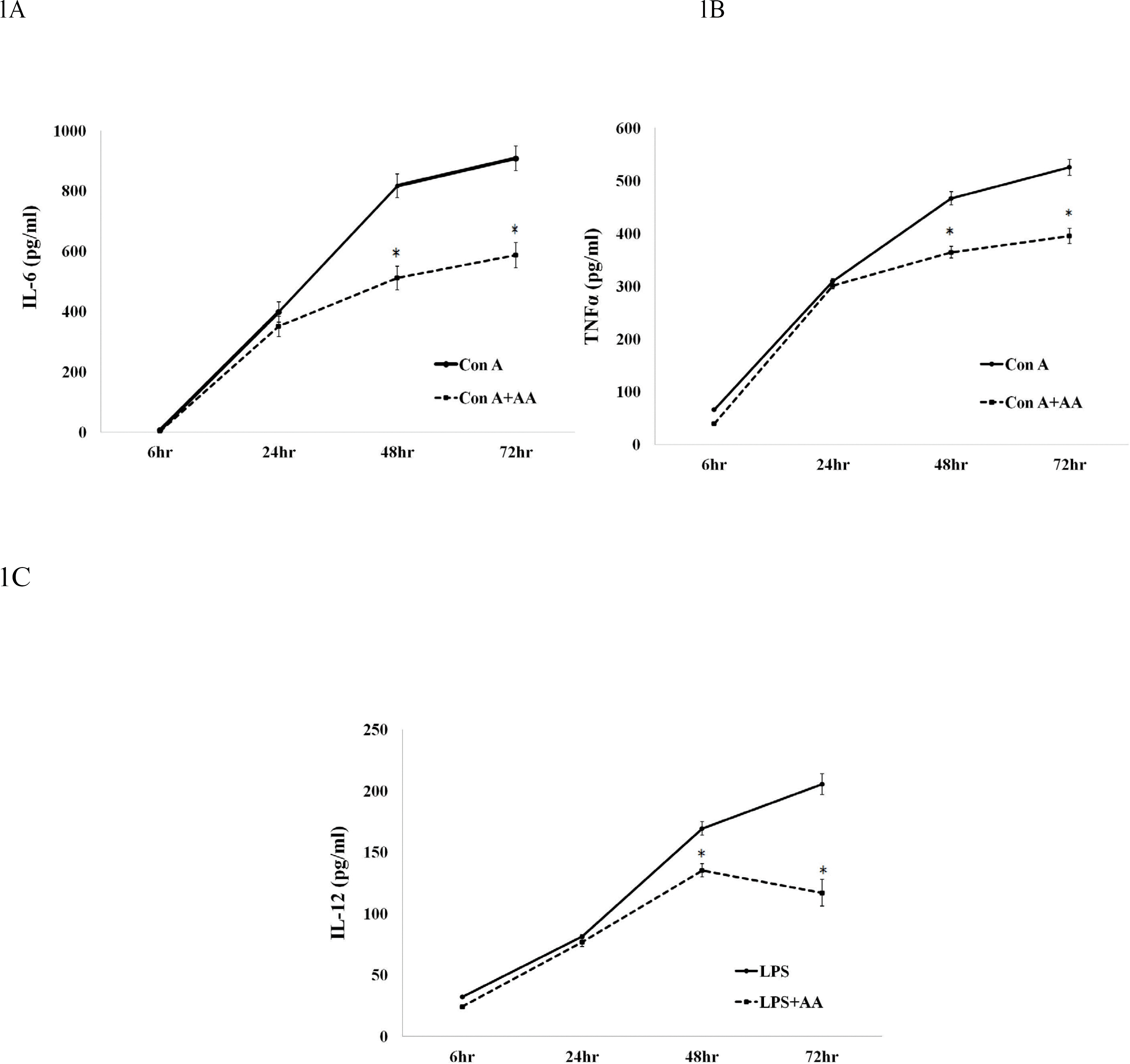
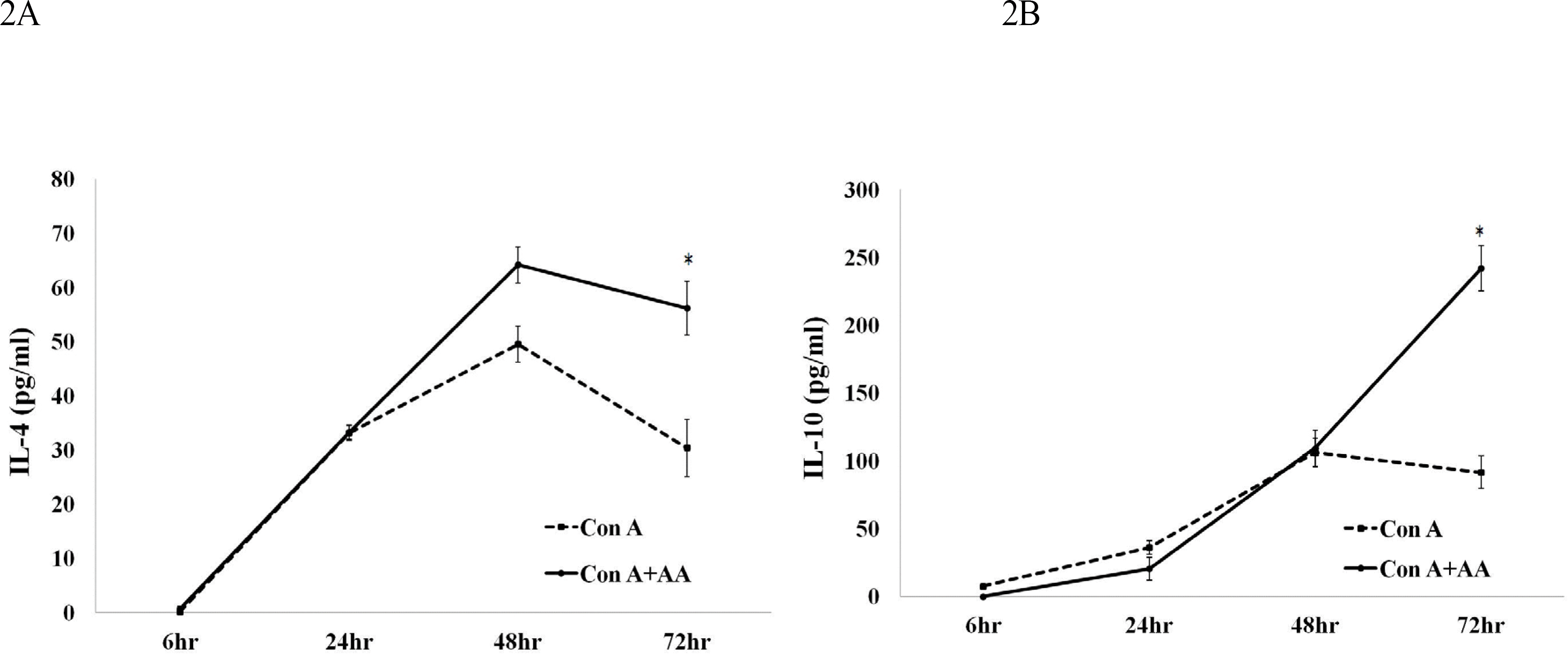
AA significantly downregulated the expression of IL-6, IL-12, and TNF-α at 48 h and 72 h in mouse splenocytes treated with a combination of polyclonal activators and AA. AA treatment also resulted in upregulation of IL-4 and IL-10 at 72 h. These findings demonstrated that AA significantly potentiated production of anti-inflammatory cytokines whereas there was an inverse association between AA and expression of pro-inflammatory cytokines in mouse splenocytes.
References
1. Verri WA Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther. 2006; 112:116–38.

2. Chen Y, Boettger MK, Reif A, Schmitt A, Uçeyler N, Sommer C. Nitric oxide synthase modulates CFA-induced thermal hyperalgesia through cytokine regulation in mice. Mol Pain. 2010; 6:13.

3. Kelley DS, Bendich A. Essential nutrients and immunologic functions. Am J Clin Nutr. 1996; 63:994S–6S.

4. Puertollano MA, Puertollano E, de Cienfuegos GA, de Pablo MA. Dietary antioxidants: immunity and host defense. Curr Top Med Chem. 2011; 11:1752–66.
5. Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in Disease Prevention and Cure: An Overview. Indian J Clin Biochem. 2013; 28:314–28.

6. Nam-Sung Kang, Suhkneung Pyo, eun-Hua sohn. In vitro Effects of L-Ascorbic Acid and Acrylamide on Lymphocyte Proliferation in Young and Aged Mice. J Food Sci Nutr. 2010; 15:19–23.
7. Pae M, Ren Z, Meydani M, Shang F, Smith D, Meydani SN, et al. Dietary supplementation with high dose of epigallocatechin-3-gallate promotes inflammatory response in mice. J Nutr Biochem. 2012; 23:526–31.

8. Cavaillon JM, Fitting C, Haeffner-Cavaillon N, Kirsch SJ, Warren HS. Cytokine response by monocytes and macrophages to free and lipoprotein-bound lipopolysaccharide. Infect Immun. 1990; 58:2375–82.

9. Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, et al. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A. 2008; 105:11105–9.

10. Moens B, Decanine D, Khouri R, Lopez G, Talledo M, Gotuzzo E, et al. Ascorbic acid has superior antiviral and antiproliferative effects over IFN-alpha in HAM/TSP PBMC ex vivo. Retrovirology. 2011; 8:A61.

11. Üçeyler N, Sommer C. Cytokine-induced pain: basic science and clinical implications. Rev Analg. 2007; 9:87–103.

12. Kress M. Nociceptor Sensitization by Proinflammatory Cytokines And Chemokines. The Open Pain Journal. 2010; 3:97–107.

13. Uceyler N, Sommer C. Cytokine regulation in animal models of neuropathic pain and in human diseases. Neurosci Lett. 2008; 437:194–8.
14. Chen H, Karne RJ, Hall G, Campia U, Panza JA, Cannon RO 3rd, et al. High-dose oral vitamin C partially replenishes vitamin C levels in patients with Type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am J Physiol Heart Circ Physiol. 2006; 290:H137–45.

15. Tousoulis D, Antoniades C, Vasiliadou C, Kourt-ellaris P, Koniari K, Marinou K, et al. Effects of atorvastatin and vitamin C on forearm hyperaemic blood flow, asymmentrical dimethylarginine levels and the inflammatory process in patients with type 2 diabetes mellitus. Heart. 2007; 93:244–6.

16. Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003; 23:2517–21.
17. Zelenka M, Schafers M, Sommer C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain. 2005; 116:257–63.
18. Cunha TM, Verri WA Jr, Valerio DA, Guerrero AT, Nogueira LG, Vieira SM, et al. Role of cytokines in mediating mechanical hypernociception in a model of delayed-type hypersensitivity in mice. Eur J Pain. 2008; 12:1059–68.

19. Barksby HE, Lea SR, Preshaw PM, Taylor JJ. The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders. Clin Exp Immunol. 2007; 149:217–25.

20. Qiu J, Cafferty WB, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci. 2005; 25:1645–53.

21. Cafferty WB, Gardiner NJ, Das P, Qiu J, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knockout mice. J Neurosci. 2004; 24:4432–43.

22. Gardiner NJ, Cafferty WB, Slack SE, Thompson SW. Expression of gp130 and leukaemia inhibitory factor receptor subunits in adult rat sensory neurones: regulation by nerve injury. J Neurochem. 2002; 83:100–9.

23. Obreja O, Biasio W, Andratsch M, Lips KS, Rathee PK, Ludwig A, et al. Fast modulation of heat-activated ionic current by proinflammatory interleukin 6 in rat sensory neurons. Brain. 2005; 128:1634–41.

24. Juttler E, Tarabin V, Schwaninger M. Interleukin-6 (IL-6): a possible neuromodulator induced by neuronal activity. Neuroscientist. 2002; 8:268–75.

25. Vollmer-Conna U, Fazou C, Cameron B, Li H, Brennan C, Luck L, et al. Production of proinflammatory cytokines correlates with the symptoms of acute sickness behaviour in humans. Psychol Med. 2004; 34:1289–97.

26. Wang XS, Shi Q, Williams LA, Cleeland CS, Mobley GM, Reuben JM, et al. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer. 2008; 113:2102–9.

27. Karam MC, Hamdan HG, Abi Chedid NA, Bodman-Smith KB, Baroody GM. Interleukin-10 reduces hyperalgesia and the level of Interleukin-1beta in BALB/c mice infected with Leishmania major with no major effect on the level of Interleukin-6. J Neuroimmunol. 2007; 183:43–9.
28. Milligan ED, Langer SJ, Sloane EM, He L, Wieseler-Frank J, O'Connor K, et al. Controlling pathological pain by adenovirally driven spinal production of the antiinflammatory cytokine, interleukin-10. Eur J Neurosci. 2005; 21:2136–48.

29. Uceyler N, Rogausch JP, Toyka KV, Sommer C. Differential expression of cytokines in painful and painless neuropathies. Neurology. 2007; 69:42–9.

30. Uceyler N, Sommer C. Status of immune mediators in painful neuropathies. Curr Pain Headache Rep. 2008; 12:159–64.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download