Abstract
Background
Pulmonary carcinoid tumors consisting of typical carcinoid tumors (TC) and atypical carcinoid tumors (AC) are rare, accounting for 2–5% of all lung tumors. TC is considered a low-grade tumor with a rate of distant metastasis up to 12%. In contrast, ACs are more aggressive tumors, displaying a metastatic rate up to 70%. Surgery is the treatment of choice; however, the current treatment outcomes of metastatic lung carcinoids are discouraging. This study aimed to investigate the EGFR mutation using the PNA-mediated clamping method and to provide basic data for using EGFR-TK1 and its clinical implications.
Materials and Methods
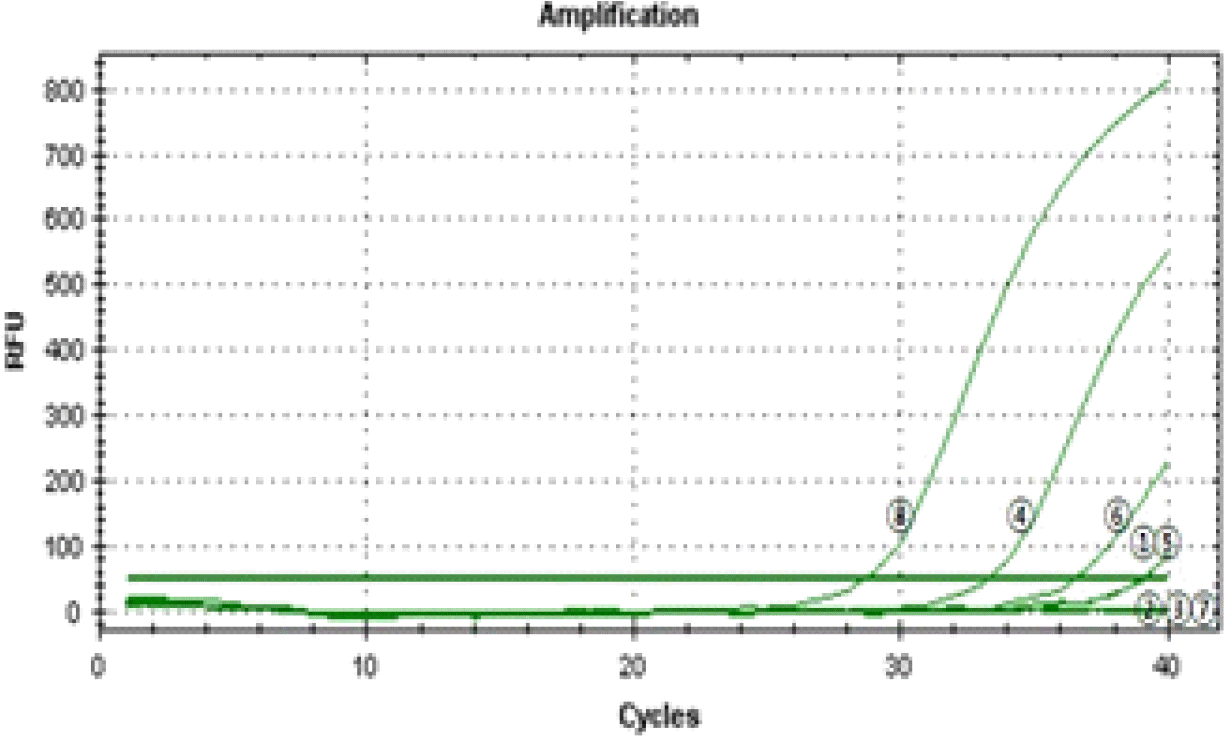
A total of 14 cases that underwent surgery were diagnosed as carcinoid tumors and pathologically classified as TC and AC. The paraffin-embedded tissues were analyzed for EGFR mutations using the PNA-mediated PCR clamping technique. The mutant type was noted in the cases with a△Ct greater than 2.0.
Go to : 
References
1. Cardillo G, Sera F, Di Martino M. Bronchial carcinoid tumors: nodal status and longterm survival after resection. Ann Thorac Surg. 2004; 77:1781–5.

2. Travis WD, Gal AA, Colby TV, Klimstra DS, Falk R, Koss MN. Reproducibility of neuroendocrine lung tumor classification. Hum Pathol. 1998; 29:272–9.

3. Kelly K, Huang C. Biological agents in non-small cell lung cancer: a review of recent advances and clinical results with a focus on epidermal growth factor receptor and vascular endothelial growth factor. J Thorac Oncol. 2008; 3:664–73.

4. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–39.

5. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 304:1497–500. 2004.
6. Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebocontrolled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 366:1527–37. 2005.

7. Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, et al. Erlotinib in lung cancer – molecular and clinical predictors of outcome. N Engl J Med. 2005; 353:133–44.

8. Zhu CQ, da Cunha Santos G, Ding K, Sakurada A, Cutz JC, et al. National Cancer Institute of Canada Clinical Trials Group Study BR.21. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2008; 26:4268–75.
10. Pao W, Ladanyl M. Epidermal growth factor receptor mutation testing in lung cancer: searching for the ideal method. Clin Cancer Res. 2007; 13:4954–5.
12. Jang TW, Oak CH, Chang HK, Suo SJ, Jung MH. EGFR and KRAS mutations in patients with adenocarcinoma of the lung. Kor J Int Med. 2009; 24:48–54.

13. Saijo N, Fukuoka M, Thongprasert S, Ichinose Y, Mitsudomi T, Mok TS, et al. Lung cancer working group report. Jpn J Clin Oncol. 2010; 40:i7–12.

14. Tanaka T, Matsuoka M, Sutani A, Gemma A, Maemondo M, Inoue A. Frequency of and variables associated with the EGFR mutation and its subtypes. Int J Cancer. 2010; 126:651–5.

15. Kim HJ, Lee KY, Kim YC, Lee SY, Jang TW, Lee MK, et al. Detection and comparison of peptide nucleic acid-mediated real time polymerase chain reaction clamping and direct gene sequencing for epidermal growth factor receptor mutations in patients with non-small cell lung cancer. Lung cancer. 2012; 75:321–5.
16. Yeo CD, Kim JW, Kim KH, Ha JH, Rhee CK, Kim SJ, et al. Detection and comparison of EGFR mutation in matched tumor tissues, cell blocks, pleural effusions, and sera from patients with NSCLC with malignant pleural effusion, by PNA clamping and direct sequencing. Lung Cancer. 2013; 81:207–12.
17. Rickman OB, Vohra PK, Sanyal B, Vrana JA, Aubry MC, Wigle DA, et al. Analysis of ErbB receptors in pulmonary carcinoid tumors. Clin Cancer Research. 2009; 15:3315–24.

18. Wheler JJ, Falchook GS, Tsimberidou AM, Hong DS, Naing A, Piha-Paul SA, et al. Aberrations in the epidermal growth factor receptor gene in 958 patients with diverse advances tumors: implications for therapy. Ann Oncol. 2013; 24:838–42.
19. Gilbert JA, Lloyd RV, Ames MM. Lack of mutations in EGFR in gastroenteropancreatic neuroendocrine tumors. N Engl J Med. 2005; 353:209–10.
20. Ameur N, Lacroix L, Motte N, Baudin E, Caillou B, Ducreux M, et al. Mutational status of EGFR, BRAF, PI3KCA and JAK2 genes in endocrine tumors. Int J Cancer. 2009; 124:751–3.
21. Jackman DM, Yeap BY, Sequist LV, Linderman N, Holmes AJ, Joshi VA. Exon 19 deletion mutation of EGFR are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006; 12:3908–14.
22. Thomas CF Jr, Tazelaar HD, Jett JR. Typical and atypical pulmonary carcinoids outcome in patients presenting with regional lymph node involvement. Chest. 2001; 119:1143–50.
23. Yanagisawa S, Morikawa N, Kimura Y, Nagano Y, Murakami K, Tabata T. Large cell neuroendocrine carcinoma with epidermal growth factor receptor mutation: possible transformation of lung adenocarcinoma. Respirology. 2012; 17:1275–7.
Go to : 
Table 1.
The clinical and pathological characteristics of 14 cases of pulmonary carcinoid tumors
Table 2.
The results of analysis of EGFR PNA-mediated clamping using real-time PCR




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download