Abstract
Objectives
The aim of this retrospective study was whether serum Tg predicts malignancy in follicular or Hürthle-cell neoplasms on fine needle aspiration.
Methods
A chart review of 111 patients (90 females, 21 males; mean age 46.8 ± 11.9 years) with follicular or Hürthle-cell neoplasms on fine needle aspiration, who were surgically treated between Sep. 2001 and Sep. 2011, was performed. Predictive factors for malignancy were identified by the chi-squared test and multivariate logistic regression.
Results
There were no differences between 41 malignant and 70 benign lesions in serum Tg or any of the normalized indexes. Receiver-operator characteristic analysis revealed no cutoff value. Lesions with serum Tg levels greater than 500 g/L had no significant difference. And also there were no independent predictors of malignancy by multivariate logistic regression.
REFERENCES
1. Vander JB, Gaston EA, Dawber TR. The significance of nontoxic thyroid nodules. Final report of a 15-year study of the incidence of thyroid malignancy. Ann Intern Med. 1968; 69:537–40.
2. Hegedüs L. Clinical practice. The thyroid nodule. N Engl J Med. 2004; 351:1764–71.
3. Tan GH, Gharib H. Thyroid incidentalomas: Management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. 1997; 126:226–31.

4. Ezzat S, Sarti DA, Cain DR, Braunstein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994; 154:1838–40.

6. Baloch ZW, Sack MJ, Yu GH, Livolsi VA, Gupta PK. Fine-needle aspiration of thyroid: an institutional experience. Thyroid. 1998; 8:565–9.

7. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008; 58:71–96.

8. Jung KW, Won YJ, Park S, Kong HJ, Sung J, Shin HR, et al. Cancer statistics in Korea: incidence, motality and surival in 2005. J Korean Med Sci. 2009; 23:995–1003.
9. Sorrenti S, Trimboli P, Catania A, Ulisse S, De Antoni E, D'Armiento M. Comparison of malignancy rate in thyroid nodules with cytology of indeterminate follicular or indeterminate Hürthle cell neoplasm. Thyroid. 2009; 19:355–60.

10. Thoresen SO, Myking O, Glattre E, Rootwelt A, Foss OP. Serum thyroglobulin as a preclinical tumour marker in subgroups of thyroid cancer. Br J Cancer. 1988; 57:105–8.

11. Petric R, Perhavec A, Gazic B, Besic N. Preoperative serum thyroglobulin concentration is an independent predictive factor of malignancy in follicular neoplasms of the thyroid gland. J Surg Oncol. 2012; 105:351–6.

13. Suh I, Vriens MR, Guerrero MA, Griffin A, Shen WT, Duh QY, et al. Serum thyroglobulin is a poor diagnostic biomarker of malignancy in follicular and Hurthle-cell neoplasms of the thyroid. Am J Surg. 2010; 200:41–6.
14. Cibas ES, Ali SZ. NCI Thyroid FNA State of the Science Conference. The Bethesda System for reporting thyroid cytopathology. Am J Clin Pathol. 2009; 132:658–65.

15. Castro MR, Gharib H. Thyroid fine-needle aspiration biopsy: progress, practice, and pitfalls. Endocr Pract. 2003; 9:128–36.

16. Cappelli C, Castellano M, Pirola I, Cumetti D, Agosti B, Gandossi E, et al. The predictive value of ultrasound findings in the management of thyroid nodules. QJM. 2007; 100:29–35.

17. Yim JH, Kim EY, Kim WG, Kim TY, Gong G, Hong SJ, et al. Postoperative Findings of the Cytological Diagnosis of Follicular Neoplasm or Hurthle Cell Neoplasm and the Risk of Malignancy. Endocrinol Metab. 2010; 25:316–20.
18. Herle AJ, Uller RP. Elevated serum thyroglobulin. A marker of metastases in differentiated thyroid carcinomas. J Clin Invest. 1975; 56:272–7.

19. Black EG, Cassoni A, Gimlette TM, Harmer CL, Maisey MN, Oates GD, et al. Serum thyroglobulin in thyroid cancer. Lancet. 1981; 29:443–5.

20. Hrafnkelsson J, Tulinius H, Kjeld M, Sigvaldason H, Jónasson JG. Serum thyroglobulin as a risk factor for thyroid carcinoma. Acta Oncol. 2000; 39:973–7.
21. Hocevar M, Auersperg M. Role of serum thyroglobulin in the pre-operative evaluation of follicular thyroid tumours. Eur J Surg Oncol. 1998; 24:553–7.

22. Okamoto T, Kanbe M, Iihara M, Yamazaki K, Okamoto J, Yamashita T, et al. Measuring serum thyroglobulin in patients with follicular thyroid nodule: its diagnostic implications. Endocr J. 1997; 44:187–93.

23. Nikola Besic, Manja Sesek, Barbara Peric, Janez Zgajnar, Marko Hocevar. Predictive factors of carcinoma in 327 patients with follicular neoplasm of the thyroid. Med Sci Monit. 2008; 14:459–67.
Fig. 1.
Box plot comparison of serum thyroglobulin values between benign and malignant neoplasms with follicular or Hürthle cell neoplasm on fine needle aspiration.
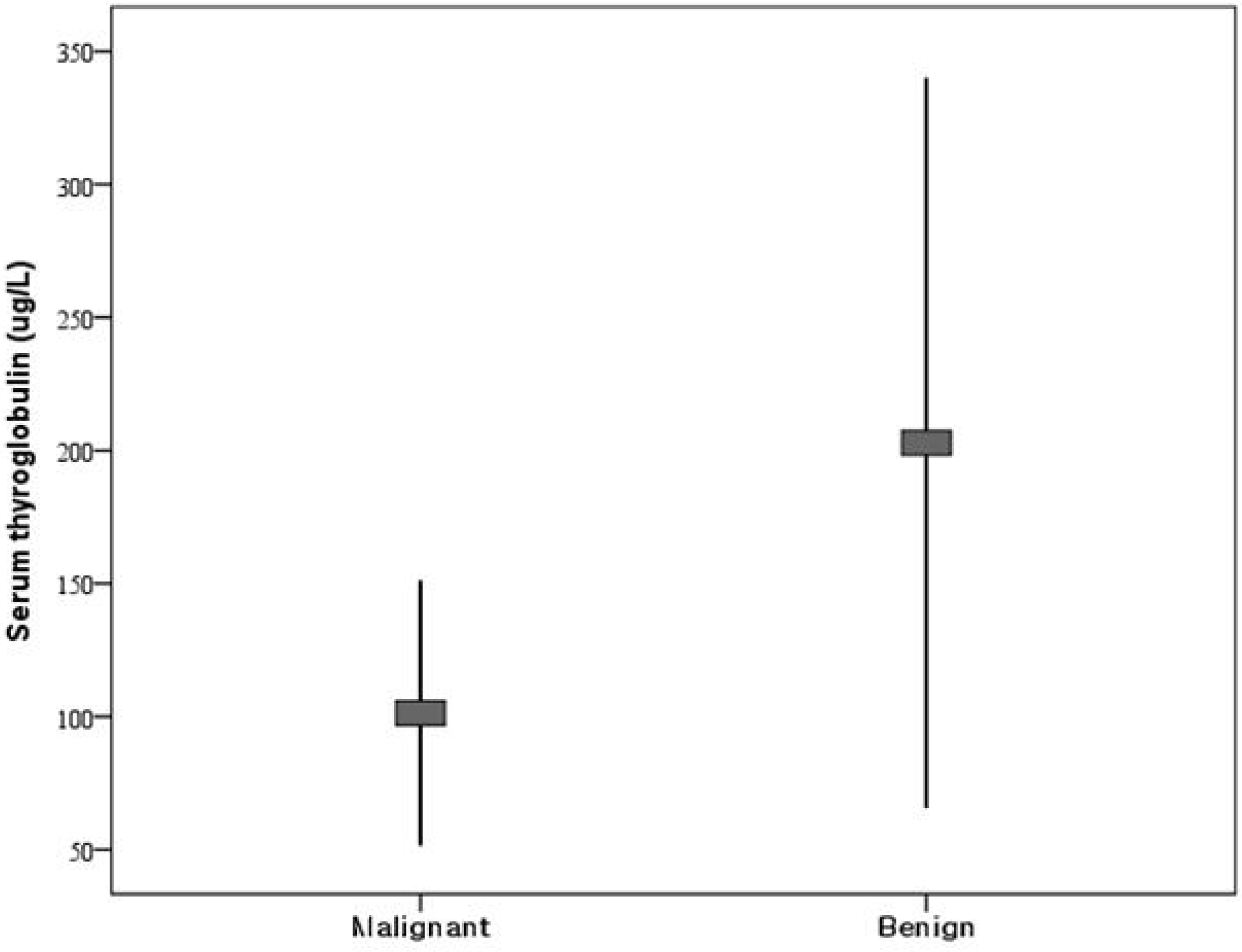
Table 1.
Clinical characteristics of study subjects
Table 2.
Histologic diagnosis of study samples
Table 3.
Comparison of malignant and benign histology groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download