Abstract
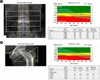
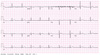
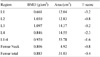
Recombinant human parathyroid hormone 1-34 (rHPTH; 1-34, teriparatide) increases bone mass and increases osteoporotic fracture by stimulating new bone formation. It was approved in the United States for treatment of osteoporosis in men and women, and its effectiveness and safety was proved. Mild hypercalcemia was observed, but persistent and severe hypercalcemia was not observed in the studies of teriparatide. In this case, severe hypercalcemia was developed from patient having gait disturbance who was treated with vitamin D, calcium and teripartide for two months to treat osteoporosis after subtrochanteric fracture. Hypercalcemia was resolved with discontinuation of teriparatide. Severe hypercalcemia is not a common complication of teriparatide and monitoring of serum calcium level is routinely not recommended. But it is necessary to pay close attention to patients who were treated with teriparatide, especially in immobilized patients.
Figures and Tables
References
1. Health NIo. Osteoporosis and related bone diseases-National Resource Center. Osteoporosis. 2011. cited by 2012 April 20. Available from: http://www.niams.nih.gov/Health_Info/Bone/Osteoporosishttp://www.niams.nih.gov/Health_Info/Bone/Osteoporosis.
2. Crandall C. Osteoporosis: A two-article symposium. Postgrad Med. 2003. 114:21.
3. Turner RT, Evans GL, Lotinun S, Lapke PD, Iwaniec UT, Morey-Holton E. Dose-response effects of intermittent PTH on cancellous bone in hindlimb unloaded rats. J Bone Miner Res. 2007. 22:64–71.

4. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001. 344:1434–1441.

5. Jüppner H, Kronenberg HM. Favus MJ, editor. Parathyroid hormone. Primer on the metabolic bone diseases and disorders of mineral metabolism. 2003. 5th ed. Washington, DC: American Society for Bone and Mineral Research;117–124.
6. Hodsman AB, Bauer DC, Dempster DW, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005. 26:688–703.

8. Carroll R, Matfin G. Review: Endocrine and metabolic emergencies: hypercalcaemia. Ther Adv Endocrinol Metab. 2010. 1:225–234.

10. DeVivo MJ, Fine PR, Cutter GR, Maetz HM. The risk of renal calculi in spinal cord injury patients. J Urol. 1984. 131:857–860.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download