Abstract
Objectives
The purpose of this study is to identify whether the change of pH affects the proliferation and the differentiation of human bone marrow stem cells (hBMSCs) and what mechanism is underlied.
Methods
To achieve objective of this study, hBMSCs were cultivated in the conditioned media adjusted to potential of hydrogen (pH) ranging from 6.4 to 8.0 using addition of hydrochloric acid (HCl) and sodium hydroxide (NaOH). The ratio of proliferation of hBMSCs according to the change of pH was measured for 24 h, 48 h, and 72 h using water-soluble tetrazolium salt (WST)-8 method. To elucidate the mechanism involved, hBMSCs was subjected to blocking extracellular signal-regulated kinases (ERK) and calcium sensing receptor (CaSR) activation. The Osteogenic-related genes and alkaline phosphatase (ALP) activity were tested under the conditioned media.
Figures and Tables
 | Fig. 1The proliferation of human bone marrow stem cells (hBMSCs) according to medium potential of hydrogen in time-course dependent manner. (A) The proliferation of hBMSCs according to medium pH for 24 h incubation. (B) The proliferation of hBMSCs according to medium pH for 48 h incubation. (C) The proliferation of hBMSCs according to medium pH for 72 h incubation. The data are presented as means ± standard error of mean (SEM). * denotes a difference at P < 0.05. |
 | Fig. 2The expression of calcium-sensing receptor (CaSR) messenger ribonucleic acid (mRNA) and protein according to medium potential of hydrogen (pH) in time-course dependent manner. (A) The expression levels of CaSR mRNA according to medium pH in time-course dependent manner. (B) The expression levels of CaSR protein according to medium pH in time-course dependent manner. (GAPDH, glyceraldehyde-3-phosphate dehydrogenase) |
 | Fig. 3The suppression of proliferation in human bone marrow stem cells (hBMSCs) exposed by calcium-sensing receptor (CaSR) inbibitor. The data are presented as means ± standard error of mean (SEM). * denotes a difference at P < 0.05. |
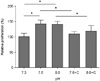 | Fig. 4The suppression of proliferation in human bone marrow stem cells (hBMSCs) exposed by extracellular signal-regulated kinases (ERK) inhibitor. The data are presented as means ± standard error of mean (SEM). * denotes a difference at P < 0.05. |
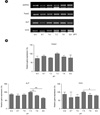 | Fig. 5The expression of osteogenic-related molecules according to medium potential of hydrogen (pH). (A) The expression levels of runt-related transcription factor Runx2, alkaline phosphatase (ALP), and osteocalcin (OCN) messenger ribonucleic acid (mRNA) according to medium pH. (B) Quantitative analysis of Rnunx2, ALP, and OCN mRNA according to medium pH (Y-axis is a normalized level). The data are presented as means ± standard error of mean (SEM). * and ** denotes a difference at P < 0.05 and P < 0.01, respectively. (GAPDH, glyceraldehyde-3-phosphate dehydrogenase) |
 | Fig. 6The activities of alkaline phosphatase (ALP) according to medium potential of hydrogen (pH). (A) Quantitative analysis of ALP activities according to medium pH for 7 days incubation. (B) Quantitative analysis of ALP activities according to medium pH for 14 days incubation (Y-axis is a normalized level). The data are presented as means ± standard error of mean (SEM). * denotes a difference at P < 0.05. |
References
1. Kaila K, Panula P, Karhunen T, Heinonen E. Fall in intracellular pH mediated by GABAA receptors in cultured rat astrocytes. Neurosci Lett. 1991. 126:9–12.

2. Ramp WK, Lenz LG, Kaysinger KK. Medium pH modulates matrix, mineral, and energy metabolism in cultured chick bones and osteoblast-like cells. Bone Miner. 1994. 24:59–73.

3. Reeve J, Arlot M, Wootton R, et al. Skeletal blood flow, iliac histomorphometry, and strontium kinetics in osteoporosis: a relationship between blood flow and corrected apposition rate. J Clin Endocrinol Metab. 1988. 66:1124–1131.

5. Kohn DH, Sarmadi M, Helman JI, Krebsbach PH. Effects of pH on human bone marrow stromal cells in vitro: implications for tissue engineering of bone. J Biomed Mater Res. 2002. 60:292–299.

6. Frick KK, Bushinsky DA. Metabolic acidosis stimulates RANKL RNA expression in bone through a cyclo-oxygenase-dependent mechanism. J Bone Miner Res. 2003. 18:1317–1325.

7. Brandao-Burch A, Utting JC, Orriss IR, Arnett TR. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif Tissue Int. 2005. 77:167–174.

8. Disthabanchong S, Radinahamed P, Stitchantrakul W, Hongeng S, Rajatanavin R. Chronic metabolic acidosis alters osteoblast differentiation from human mesenchymal stem cells. Kidney Int. 2007. 71:201–209.

9. Brown EM, Gamba G, Riccardi D, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993. 366:575–580.

10. Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001. 81:239–297.

11. Maiti A, Hait NC, Beckman MJ. Extracellular calcium-sensing receptor activation induces vitamin D receptor levels in proximal kidney HK-2G cells by a mechanism that requires phosphorylation of p38alpha MAPK. J Biol Chem. 2008. 283:175–183.

12. Yamaguchi T, Chattopadhyay N, Kifor O, Butters RR Jr, Sugimoto T, Brown EM. Mouse osteoblastic cell line (MC3T3-E1) expresses extracellular calcium (Ca2+o)-sensing receptor and its agonists stimulate chemotaxis and proliferation of MC3T3-E1 cells. J Bone Miner Res. 1998. 13:1530–1538.

13. Yamaguchi T, Kifor O, Chattopadhyay N, Brown EM. Expression of extracellular calcium (Ca2+o)-sensing receptor in the clonal osteoblast-like cell lines, UMR-106 and SAOS-2. Biochem Biophys Res Commun. 1998. 243:753–757.

14. Yamaguchi T, Chattopadhyay N, Kifor O, et al. Expression of extracellular calcium-sensing receptor in human osteoblastic MG-63 cell line. Am J Physiol Cell Physiol. 2001. 280:C382–C393.

15. Chang W, Tu C, Chen TH, et al. Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology. 1999. 140:5883–5893.

16. Traynelis SF, Hartley M, Heinemann SF. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science. 1995. 268:873–876.

17. Quinn SJ, Bai M, Brown EM. pH Sensing by the calcium-sensing receptor. J Biol Chem. 2004. 279:37241–37249.

18. Sugimoto T, Kanatani M, Kano J, et al. Effects of high calcium concentration on the functions and interactions of osteoblastic cells and monocytes and on the formation of osteoclast-like cells. J Bone Miner Res. 1993. 8:1445–1452.

19. Quarles LD, Hartle JE 2nd, Siddhanti SR, Guo R, Hinson TK. A distinct cation-sensing mechanism in MC3T3-E1 osteoblasts functionally related to the calcium receptor. J Bone Miner Res. 1997. 12:393–402.

20. Ludwig MG, Vanek M, Guerini D, et al. Proton-sensing G-protein-coupled receptors. Nature. 2003. 425:93–98.

21. Nishida E, Gotoh Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem Sci. 1993. 18:128–131.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download