Abstract
Objectives
Osteoclasts are multinucleated giant cells which can resorb bone and differentiated from hematopoietic cells. We have previously reported murine osteoclast-associated receptor (OSCAR) may be an important bone-specific regulator of osteoclast differentiation. We have cloned soluble form of human OSCAR (hOSCAR) and examined the role of hOSCAR on osteoclast differentiation.
Methods
Osteoclast differentiation was induced by treatment with macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa B ligand (RANKL) and tartrate-resistant acid phosphatase (TRAP) staining and pit formation were performed. Expression was measured by flow cytometry analysis, Northern and Western blot analysis.
Results
hOSCAR is expressed in osteoclast cells and involved in the differentiation of osteoclasts from peripheral blood mononuclear cells (PBMC). Two alternatively spliced forms (soluble hOSCAR [hOSCAR-S]) of hOSCAR were identified from osteoclasts complementary deoxyribonucleic acid (cDNA) library derived from PBMC. Putative transmembrane domain was not found in hOSCAR-S forms and it suggested that these forms might be secreted from osteoclast cells. These secreted forms of hOSCAR attenuated RANKL-induced osteoclast formation and bone resorption.
Bone is a physiologically dramatic tissue which provides a mechanical support, physical protection, and storage site for systemic mineral homeostasis. Bone is continuously remodeled and balanced through bone formation by osteoblasts and bone resorption by osteoclasts. Osteoblasts are responsible for mineralization of bone matrix. Osteoclasts are the unique cells which can resorb mineralized bone and differentiated from hematopoietic cells.
Two essential factors, macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa B ligand (RANKL), are produced by osteoblasts and can support osteoclast differentiation from monocyte-macrophage lineage cells.1 The spontaneous mutant op/op mice, defective in M-CSF, show osteopetrotic phenotype due to the defective of differentiation of osteoclasts.2 It has been shown that RANKL, expressed on the surface of osteoblasts, is an essential molecule mediating its signals to osteoclast precursors for their differentiation into mature osteoclasts.3-6
RANKL regulates various transcription factors including nuclear factor kappa B (NF-κB), c-Fos, and nuclear factor of activated T cells (NFAT) c1, which act as positive modulators in osteoclast differentiation.1,7 Costimulatory signals mediated by immunoreceptor tyrosine-based activation motif (ITAM)-harboring adaptors, including DNAX-activating protein (DAP) 12 and Fc receptor common gamma (FcRγ) chain cooperate with RANKL during osteoclastogenesis, and their activation enhances the induction of NFATc1 via calcium signaling.8
We have shown that a novel member of leukocyte receptor complex-encoded protein osteoclast-associated receptor (OSCAR), which is expressed in osteoclasts specifically, regulates the differentiation of osteoclasts.9 OSCAR is a member of the immunoglobulin-like surface receptor family and plays an important role as a costimulatory receptor for osteoclast differentiation by activating NFATc1 via association with the FcRγ chain.8,10 In humans, OSCAR is expressed by macrophages, monocytes, and monocyte-derived dendritic cells and modulates the response of the innate and adaptive immune systems by promoting cell activation and maturation, Ag presentation, and proinflammatory circuits.11-14 Human studies indicate that OSCAR may contribute to the pathogenesis and severity of osteoporosis and rheumatoid arthritis.11-14 In this study, we found novel alternatively spliced forms of human OSCAR (hOSCAR) in osteoclasts. The aim of this study was to examine the role of alternatively spliced forms of hOSCAR on osteoclast differentiation.
All cell culture media and supplements were obtained from Gibco (Grand Island, NY, USA). Soluble recombinant mouse RANKL was purified from insect cells as described3 and recombinant human M-CSF was obtained from Genetics Institute (Cambridge, MA, USA). TRIzol and Oligotex poly A+ RNA column were purchased from Life Technologies (Grand Island, NY, USA) and QIAGEN (Valencia, CA, USA), respectively. Ready-to-Go labeling kit and ProbeQuant G-50 purification kit were from Amersham Pharmacia Biotech (Piscataway, NJ, USA). ZAP cDNA synthesis kit, Pfu DNA polymerase, and pIRES-hrGFP-1a mammalian expression vector were purchased from Agilent Technologies Inc. (Santa Clara, CA, USA). Antibodies specific for FLAG epitope (M2), biotinylated anti-FLAG epitope (BioM2), and anti-FLAG-M2 agarose bead were from Sigma-Aldrich Co. (St. Louis, MO, USA); mouse IgG-conjugated horseradish peroxidase from Amersham Pharmacia Biotech; and streptoavidin-conjugated APC from BD PharMingen (San Diego, CA, USA).
Human monocytes were isolated from peripheral blood by counter flow centrifugation. Cells from fraction 180 and 190 (more than 90% are CD14+) were used for generating macrophage (M), osteoclast (OC), and dendritic cells (DC). The fractionated cells (5 × 106) were incubated with M-CSF (30 ng/mL) alone for M, with M-CSF (30 ng/mL) and RANKL (200 ng/mL) for OC in 100 mm culture dish using alpha minimum essential medium (α-MEM) containing 10% fetal bovine serum (FBS) for 6 days. After 3 days culture, media containing macrophage-stem cell factor (M-SCF) or M-CSF and RANKL were changed freshly. The generation of DC from peripheral blood mononuclear cells (PBMC) used the method originally as described.15 To generate DC, the purified human monocytes (2 × 108) in 30 mL AIM-V (Invitrogen, Carlsbad, CA, USA) were plated in a T-150 flask, and incubated for 2 hr at 37℃ in 5% carbon dioxide (CO2) incubator. Then, non- or semiadherent cells were discarded by gentle pipetting. Adherent monocytes were cultured for 7 days with 30 mL AIM-V medium, supplemented with human granulocyte-macrophage colony-stimulating factor (hGM-CSF, 5 ng/mL) and human interleukin-4 (hIL-4, 10 ng/mL; R&D system, Minneapolis, MN, USA).
Total ribonucleic acid (RNA) was harvested from human monocyte-drived osteoclast cells using TRIzol after incubation with M-CSF and RANKL for 6 days. Poly A+ RNA was prepared from total RNA using Oligotex poly A+ RNA column. Human osteoclast complementary deoxyribonucleic acid (cDNA) library was synthesized using ZAP cDNA synthesis kit according to the manufacturer's instructions. The ecto domain of hOSCAR was amplified by polymerase chain reaction (PCR) to screen the full length cDNA clones and 13 clones of hOSCAR cDNA were picked up and analyzed.
Total RNA samples were separated and transferred to nylon membranes as described.16 Hybridization was performed at 42℃ for 16 hr with 32P-deoxycytidine triphosphate (dCTP) DNA probe prepared using Ready-to-Go labeling kit and ProbeQuant G-50 purification kit. After washing with 0.1 × saline sodium citrate (SSC) and 0.1% sodium dodecyl sulfate (SDS) at 60℃ for 1 hr, membrane was exposed to X-ray film.
Three different FLAG epitope-tagged hOSCAR constructs were amplified by PCR using Pfu DNA polymerase. To make each construct, the following primers were used: FLAG, 5'-ATA AGA ATG CGG CCG CAC CAT GTC TGC ACT TCT G-3' (sense) and 5'-CGG GAT CCG AAG CTT GTC GTC ATC GTC TTT-3' (antisense); hOSCAR-I, 5'-CGG GAT CCC CCC CAG CTT CAT ACC ACC CTA A-3' (sense) and 5'-CCG CTC GAG CGG GGG GCG GAT ACC AGC AGG AGC-3' (antisense); hOSCAR-II, 5'-CGG GAT CCC CCC CAG CTT CAT ACC ACC CTA A-3' (sense) and 5'-CCG CTC GAG CGG GAC TCC TGG ATC TGA GGG AGG A-3' (antisense); hOSCAR-V, 5'-CGG GAT CCC CCC CAG CTT CAT ACC ACC CTA A-3' (sense) and 5'-CCG CTC GAG ATT CAG CAG GAC TGT GGG GCT GCA GGA-3' (antisense). The amplified FLAG epitope fragment was digested with NotI and BamHI. The PCR products of hOSCAR-I, -II, and -V were digested with BamHI and XhoI, respectively. The digested FLAG epitope and each hOSCAR fragment were ligated into pIRES-hrGFP vector using NotI and XhoI sites. The FLAG epitope and full length of each hOSCAR were confirmed by sequencing.
Two hundred ninety-three T cells were transfected using FuGENE 6 (Roche Applied Sciences, Indianapolis, IN, USA). All cells and media were harvested after 36 hr incubation. A half of the cells were lysed in extraction buffer (10 mM Tris, potential of hydrogen [pH] 7.5, 150 mM sodium chloride [NaCl], 0.4 mM etylenediaminetetraacetic acid [EDTA], 1% Triton X-100, 1 mM phenylmethylsulfonylfluoride, 1 µg/mL leupeptin, and 0.1 U/mL aprotinin) and cleared by centrifugation to obtain the whole-cell extracts. Whole cell extracts and filtered media were incubated with anti-FLAG M2 agarose beads at 4℃ for 1 hr on rocker table and washed 3 times with extraction buffer. The immunoprecipitates were then subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidene fluoride membrane. The membrane was blocked with 5% nonfat milk in phosphate-buffered saline containing 0.1% Tween 20 at room temperature for 1 hr and incubated with 1 µg/mL of anti-FLAG M2 antibody at 4℃ for overnight. After thorough washing, the membrane was incubated with anti-mouse IgG-conjugated horseradish peroxidase antibody at room temperature for 1 hr. Membrane was developed with enhanced chemiluminescence (ECL) western blotting detection systems.
The other half of the transfected cells were used for flow cytometry analysis. The expression of FLAG epitope-tagged hOSCAR on the surface of the cells was assessed using biotinylated anti-FLAG antibody (5 µg/mL) and followed by streptoavidin-conjugated APC.
The extracellular domain of hOSCAR (amino acid 1-219 of soluble hOSCAR [hOSCAR-S]1) was fused to Fc region of hIgG1. The soluble hOSCAR-Fc was expressed and purified from insect cells as described.16
Human peripheral blood monocytes were cultured in the presence of M-CSF (30 ng/mL) and RANKL (200 ng/mL) with hOSCAR-Fc (30 µg/mL), hIgG1 (30 µg/mL), or RANK-Fc (5 µg/mL) in 96 well culture plates or dentine slices. TRAP staining and pit formation assay were performed as described.16
We have shown that mouse OSCAR (mOSCAR) and hOSCAR have three different transcripts by alternative splicing.16 hOSCAR as well as mOSCAR has a putative signal sequence, an extracellular region consisting of two Ig-like domains, a single transmembrane region, and a short intracellular domain (Fig. 1, 2). Here we report that there are two more transcripts by alternative splicing in case of hOSCAR. The previously reported three hOSCAR transcripts are membrane-bounded forms (membrane-bound hOSCAR [hOSCAR-M]1, M2, and M3) which leader sequence region has a variation.16 The two new transcripts (hOSCAR-S1 and S2) showed variations in a leader sequence region and C-terminus region (transmembrane and cytoplasmic domains) (Fig. 1, 2). These new transcripts have exon 4b region which are spliced out in the hOSCAR-M.
Among these new transcripts, hOSCAR-S2 form represents a deletion in exon 2 like as hOSCAR-M2 form, but hOSCAR-S1, a major transcript of hOSCAR, has a deletion in only exon 2b region like as hOSCAR-M1 form.
By searching the human genomic database with hOSCAR cDNA sequence, we found a human bacterial artificial chromosome (BAC) clone, AC009968.6, containing the full span of hOSCAR gene. hOSCAR gene was located in chromosome 19q13.4, which contains in the leukocyte receptor complex (LRC). The full hOSCAR gene consists of 4 exons spaning over 6 Kbps (Fig. 1). Thus, we could determine the genomic structure of hOSCAR and its 5' flanking region. All sequences at the exon-intron junctions followed to the GT/AG rules. Exon1 and 2 encoded a leader sequence, where exon 3 and 4a encoded two Ig-like domains. Exon 4c encoded a transmembrane domain and contained a stop codon and a 3'-untranslated region. Exon 4b was spliced out by alternative splicing in hOSCAR-M forms, not in hOSCAR-S forms. We were unable to find a putative transmembrane domain in exon 4b. In case of new transcripts, there is another stop codon in exon 4b region which upstream of the transmembrane domain of membrane-bounded forms (Fig. 1).
To investigate the expression of hOSCAR gene, Northern blot analyses were performed (Fig. 3). Human monocyte cells were isolated from peripheral blood by counter flow centrifugation. More than 90% of the cells from fractions between 180 and 190 were CD14+ (data not shown). These cells were incubated with M-CSF and RANKL for the indicated days until day 8. Two major transcripts, 2.0 Kb and 1.5 Kb, were detected by probe exon 3, which is a common extracellular domain. The expression levels of those transcripts were increased until day 6 and decreased (Fig. 3A). It implicated that hOSCAR is highly expressed in mature osteoclasts, even though it is expressed in low level in human monocyte precursor cells. Since there hOSCAR-M forms don't have exon 4b, we used exon 4b region as a probe. It showed that exon 4b is specific for large transcript of hOSCAR. To check the specificity of OSCAR gene expression, we used mRNA from human monocytes, monocyte-derived mature osteoclast, macrophage, and dendritic cells for Northern blot analysis. OSCAR as well as TRAP was highly expressed in mature osteoclast (Fig. 3B). When we used mRNA from the various tissues, the expression of hOSCAR and TRAP genes were not detected (Fig. 3C).
To characterize the hOSCAR-S isoforms, we performed a transient transfection experiment in 293T cells using three different constructs of hOSCAR-I, -II, and -V forms (Fig. 4). Cell lysates and supernatants were pulled down with anti-FLAG antibody and probed with anti-FLAG antibody after SDS-PAGE. The strong signal of hOSCAR-I form (FLAG-tagged hOSCAR-M form) was detected in cell lysate and hOSCAR-II and -V forms (FLAG-tagged hOSCAR-S form) showed bands in supernatants. In consistency, FACS analysis with those transiently transfected 293T cells revealed that OSCAR is highly expressed on the cell surface in hOSCAR-I form-transfected but not in hOSCAR-II and -V forms, although some minor expression was detected on the surface of hOSCAR-V. These data implicate that hOSCAR-S forms can be mainly secreted from osteoclasts and hOSCAR-M forms are expressed on the surface of osteoclasts.
Since we already showed mOSCAR is involved in the differentiation of osteoclast precursors to mature, multinucleated osteoclasts. We examined the role of hOSCAR in human monocyte culture using a soluble form of OSCAR, hOSCAR-Fc, which is made by fusing the extracellular domain of OSCAR to the Fc portion of human IgG1. When hOSCAR-Fc was added to human monocyte culture in the presence of M-CSF and RANKL, the formation of TRAP (+) multinucleated osteoclasts (MNCs) was significantly inhibited (Fig. 5). Furthermore, when cultures were made on dentin slice, hOSCAR-Fc inhibited significantly bone resorption (Fig. 5). These results indicate that OSCAR is required for the differentiation of mature osteoclasts derived from PBMC in the presence of M-CSF and RANKL.
Osteotropic factors can regulate the differentiation into multinucleated osteoclasts from hematopoetic cells, the survivability of matured osteoclasts, and resorption of bone. Soluble forms of M-CSF and RANKL can support the osteoclastogenesis from mouse bone marrow or human monocyte cells.5,6 In addition to these essential molecules, we have identified a new gene OSCAR which is involved in osteoclastogenesis.16
Here we reported that new isoforms of hOSCAR (hOSCAR-S) which are dominant forms in cDNAs. These forms also showed variations in N-terminus region like as hOSCAR-M forms. But, these new isoforms have another part of exon, exon 4b, which is spliced out by alternative splicing in hOSCAR-M forms. Since exon 4b has a new stop codon in the upstream of transmembrane domain (exon 4c) and doesn't have putative transmembrane domain, we tested whether these forms can be secreted or not in 293T cells. Two hundred ninety-three T-transient experiments show that hOSCAR-S forms are abundantly secreted to the outside of the cells, even though some populations of the cells show a low level of expression of hOSCAR on the surface of the cells. It suggests that exon 4b, which is specific for hOSCAR-S forms, makes them secret from osteoclast cells. Northern blot analysis show that large transcript of OSCAR is abundantly expressed in osteoclasts, which suggests that hOSCAR-S might be a major forms in human osteoclasts. In contrast, large transcript of mOSCAR is majorly expressed in mouse osteoclasts like as human, but it is because of longer 3'-untranslated region.16 We could not find any secreted forms of mouse OSCAR cDNA.
Our data indicate that hOSCAR is also important for the differentiation of mature osteoclasts from PBMC. It could be hypothesized that hOSCAR-S forms interfere with binding to OSCAR ligand and thus acts as a negative regulator of hOSCAR-M forms. Even though extracellular domains, domain 1 and 2, are common in membrane-bounded and secreted forms of hOSCAR, the C-terminus of hOSCAR-S forms is different from that of hOSCAR-M forms. It could not be ruled out that secreted forms might have a different role from membrane-bounded forms of hOSCAR.
The expression patterns by Northern blot analysis show that hOSCAR is highly expressed in mature osteoclasts like as mOSCAR whereas significantly lower level of hOSCAR as well as TRAP were found in human monocyte, monocyte-derived macrophage, and dendritic cells. Even though we added RANKL-receptor (RANK)-Fc into cell culture during the differentiation of macrophage or dendritic cells to block the activity of RANKL which might be secreted by some cells of monocyte mixtures, the expression of OSCAR and TRAP were still detected. We can not rule out that small amount of the other osteotropic factor such as TNF-α might be secreted by some cells. When mRNA prepared from different human tissue was analyzed, expressions of hOSCAR and TRAP showed very low levels or negative in all tested soft tissue. However, under more sensitive condition, hOSCAR was detected in peripheral blood leukocyte, bone marrow, and lung (data not shown).
In conclusion, human osteoclasts express at least five different OSCAR mRNA isoforms which could play different regulatory roles for differentiation. The secreted forms of hOSCAR might be a negative regulator of membrane-bounded forms of OSCAR. The mechanism of gene regulations of OSCAR will be needed to study in detail.
Figures and Tables
 | Fig. 1
Genomic structure and isoforms of human osteoclast-associated receptor (hOSCAR). Four exons and three introns are shown as boxes and lines, respectively. The numbers of nucleotide sequences are indicated in the parentheses. The potential transmembrane domain (TM) and stop codons are indicated with triangle. The numbers of amino acid and transcript sizes of hOSCAR isoforms are indicated. hOSCAR-M, membrane-bound human osteoclast-associated receptor; hOSCAR-S, soluble human osteoclast-associated receptor. |
 | Fig. 2
Sequence analysis of human osteoclast-associated receptor (hOSCAR). The amino acid sequences of hOSCAR isoforms are shown as one letter code. Sequences are divided into each putative region: N-termini, domain 1, domain 2, and C-termini. The potential transmembrane domain is in boldface and underlined letters. All isoforms have common domain 1 and 2. GeneBank accession numbers for hOSCAR isoforms are AF391162, AF391163, AF391164, AF474152, and AF474153. |
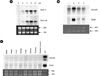 | Fig. 3
Human osteoclast-associated receptor (hOSCAR) messenger ribonucleic acid (mRNA) expression. (A) hOSCAR mRNA expression during the differentiation of peripheral blood mononuclear cells (PBMC) to mature osteoclasts by macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa B ligand (RANKL). Northern blot analysis was performed using total RNAs for 0 to 8 days. OSCAR exon 3 and 4b were used for probes. (B) Northern blot analysis of RNAs from PBMC (lane 1), PBMC-derived osteoclasts (lane 2), macrophages (lane 3), and dendritic cells (lane 4). (C) Northern blot analysis of OSCAR in various human tissues and PBMC-derived osteoclasts. Twenty-eight S and 18S of ribosomal RNAs are shown as control. |
 | Fig. 4
Transient expression of human osteoclast-associated receptor (hOSCAR) isoforms in 293T cells. (A) FLAG-tagged human OSCAR isoforms (hOSCAR-I, II, and V) were synthesized from hOSCAR-M1 or -S1 forms. (B) Western blot analysis of 293T cells transiently transfected with human OSCAR isoforms, Lane 1; control vector, 2; hOSCAR-I, 3; hOSCAR-II, and 4; hOSCAR-V. Cell lysates and supernatants were collected and immunoprecipitated with anti-FLAG antibody. Samples were resolved on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and probed with anti-FLAG antibody. (C) Flow cytomety analysis of 293T cells transfected with human OSCAR isoforms. The transfected cells were stained with biotinylated anti-FLAG antibody, followed streptoavidin-conjugated adenomatous polyposis coli (APC) and analyzed by flow cytometry. Dot line indicates vector control transfectant and solid lines indicate hOSCAR-I, -II, and -V isoforms transfectants. |
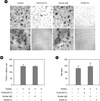 | Fig. 5
Role of human osteoclast-associated receptor (hOSCAR) in human osteoclasts differentiation. (A) Human peripheral blood mononuclear cells (PBMC) were cultured in the presence of macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa B ligand (RANKL) with 30 µg/mL hOSCAR-Fc, 30 µg/mL human IgG, or 5 µg/mL RANK-Fc in 96-well culture plates (top) or on dentine slices placed in 96-well culture plates (bottom). Cultured cells were fixed and stained for TRAP (top). Cells on dentine slices were removed, and dentine slices were stained with Mayer's hematoxylin. Pits appeared as dark spots (bottom). (B) Number of tartrate-resistant acid phosphatase (TRAP)-positive multinucleated cells (MNCs) in A was counted as osteoclasts (OCs). (C) Number of pits formed on dentine slices in A was counted. Data represent means ± SDs of triplicate samples. The results are representative of at least three independent sets of similar experiments. |
References
1. Walsh MC, Kim N, Kadono Y, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006. 24:33–63.

2. Marks SC Jr, Lane PW. Osteopetrosis, a new recessive skeletal mutation on chromosome 12 of the mouse. J Hered. 1976. 67:11–18.

3. Wong BR, Josien R, Lee SY, et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997. 186:2075–2080.

4. Anderson DM, Maraskovsky E, Billingsley WL, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997. 390:175–179.

5. Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998. 95:3597–3602.

6. Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998. 93:165–176.

7. Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999. 20:345–357.

8. Koga T, Inui M, Inoue K, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004. 428:758–763.

9. Ishida N, Hayashi K, Hoshijima M, et al. Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J Biol Chem. 2002. 277:41147–41156.

10. Ishikawa S, Arase N, Suenaga T, et al. Involvement of FcRgamma in signal transduction of osteoclast-associated receptor (OSCAR). Int Immunol. 2004. 16:1019–1025.

11. Herman S, Müller RB, Krönke G, et al. Induction of osteoclast-associated receptor, a key osteoclast costimulation molecule, in rheumatoid arthritis. Arthritis Rheum. 2008. 58:3041–3050.

12. Merck E, de Saint-Vis B, Scuiller M, et al. Fc receptor gamma-chain activation via hOSCAR induces survival and maturation of dendritic cells and modulates Toll-like receptor responses. Blood. 2005. 105:3623–3632.

13. Merck E, Gaillard C, Gorman DM, et al. OSCAR is an FcRgamma-associated receptor that is expressed by myeloid cells and is involved in antigen presentation and activation of human dendritic cells. Blood. 2004. 104:1386–1395.

14. Merck E, Gaillard C, Scuiller M, et al. Ligation of the FcR gamma chain-associated human osteoclast-associated receptor enhances the proinflammatory responses of human monocytes and neutrophils. J Immunol. 2006. 176:3149–3156.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download