Abstract
Lipoprotein receptor-related protein (LRP5) signaling is well correlated with the bone mass in both human and mice. Loss-of-function mutations of LRP5 result in osteopenia or osteoporosis. In contrast, gain-of-function mutations show high bone mass phenotype. To elucidate the molecular mechanism of the LRP5-mediated bone mass acquisition, several groups have genetically dissected the Wingless and Int-1 (Wnt)β-catenin signaling pathway using osteoblast-lineage specific Cre mice. Key players for LRP5-mediated bone mass acquisition turn out to be different molecules with respect to the expressing tissue and action mode of these molecules. One is serotonin, a tryptophan metabolite that originates from duodenum, which acts as a negative regulator for bone formation. LRP5 suppresses serotonin biosynthesis by inhibiting the expression of tryptophan hydroxylase 1 in the gut. The other is sclerostin, an osteocyte-producing antagonist for LRP5 signaling. Here is a summary of recent findings about these two molecules, providing a chance to speculate new avenues in the LRP5-mediated bone mass acquisition.
Figures and Tables
Fig. 1
Lipoprotein receptor-related protein 5 (LRP5) and Wingless and Int-1 (Wnt) signaling pathway. Wnt signaling pathway consists of β-catenin-dependent canonical and noncanonical pathways (not shown). Binding of Wnt to its receptor (Fz) and coreceptor (LRP) allows recruiting of Dishevelled (DV) and Axin (AX) to Fz and LRP5/6/4, respectively, and results in stabilization of β-catenin. Extracellular portion of LRP5/6 can bind to various proteins like Sclerostin (Sost), Wise, R-spondin (Rspo) and Dickkopf (Dkk). Complex formation of Dkk with Kremen and LRP5/6 allow to internalization of complex into cells.
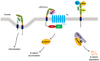
Fig. 2
Comparision of Lipoprotein receptor-related protein (LRP)4/5/6 amino acid sequences. LRP5 has a putative transmembrane sequence as signal peptide by search program (see in the text), which was not revealed in LRP4 and LRP6 (A). LRP5/6/4 show similar structure with 4 propellers in extracellular portion (B). Analyses of amino acid sequences of LRP5/6/4 show a high identity and similarity between LRP5 and LRP6 (C). LRP5, LRP6 and LRP4 belong to low density lipoprotein receptor (LDLR). Although LRP5/6/4 show similar structure and function, knockout of each gene reveals different phenotypes, which indicates that there is no compensatory function among them.

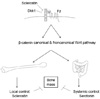
Fig. 3
Bone mass regulation in Lipoprotein receptor-related protein (LRP)5 signaling pathway via Serotonin and Sclerostin. Regulation of bone mass by LRP5 mutations is not directly correlated with the involvement of β-catenin mediated canonical Wnt pathway. High bone mass phenotypes of LRP5 gain-of-function mutations were explained by two different ways. One is local regulatory role of Sclerostin and Dickkopf (Dkk1) which are produced in bone cells and play as strong inhibitors for bone formation. They cannot bind to LRP5 with high bone mass mutations (left panel). Another explanation is systemic regulatory role of Serotonin which is produced in intestine and also play as strong inhibitor for bone formation. LRP5 gain-of-function mutations decreases blood Serotonin level by inhibition of trypthopan hydroxylase 1 expression in enterochromaffiin cells. Serotonin decreases osteoblast number and function through Serotonin receptor (Htr1b) in osteoblasts (right panel).
References
1. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011. 377:1276–1287.

2. Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007. 148:2635–2643.

3. Zhu D, Kang Q, Huang PY, He TC, Xie P. Neurogenesis-related genes expression profiling of mouse fibroblastic stem cells induced by Wnt signaling. Neurol Res. 2009. 31:200–203.

4. Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004. 20:781–810.

5. Mikels A, Minami Y, Nusse R. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J Biol Chem. 2009. 284:30167–30176.

6. He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004. 131:1663–1677.

7. Pan W, Choi SC, Wang H, et al. Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science. 2008. 321:1350–1353.

8. Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008. 133:340–353.

9. Takada I, Mihara M, Suzawa M, et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol. 2007. 9:1273–1285.

10. Maeda K, Kobayashi Y, Udagawa N, et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med. 2012. 18:405–412.

11. Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006. 25:7469–7481.

12. Gong Y, Slee RB, Fukai N, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001. 107:513–523.
13. Little RD, Carulli JP, Del Mastro RG, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002. 70:11–19.

14. Johnson ML, Gong G, Kimberling W, Reckér SM, Kimmel DB, Recker RB. Linkage of a gene causing high bone mass to human chromosome 11 (11q12-13). Am J Hum Genet. 1997. 60:1326–1332.

15. Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002. 346:1513–1521.

16. Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004. 5:691–701.
17. Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005. 8:727–738.

18. Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005. 8:739–750.

19. Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005. 132:49–60.

20. Glass DA 2nd, Bialek P, Ahn JD, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005. 8:751–764.

21. Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006. 133:3231–3244.

22. Holmen SL, Zylstra CR, Mukherjee A, et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005. 280:21162–21168.
23. Kramer I, Halleux C, Keller H, et al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010. 30:3071–3085.

24. Kato M, Patel MS, Levasseur R, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002. 157:303–314.

25. Fujino T, Asaba H, Kang MJ, et al. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc Natl Acad Sci U S A. 2003. 100:229–234.

26. Choi HY, Dieckmann M, Herz J, Niemeier A. Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS One. 2009. 4:e7930.

27. Kubota T, Michigami T, Sakaguchi N, et al. Lrp6 hypomorphic mutation affects bone mass through bone resorption in mice and impairs interaction with Mesd. J Bone Miner Res. 2008. 23:1661–1671.

28. Glass DA 2nd, Karsenty G. In vivo analysis of Wnt signaling in bone. Endocrinology. 2007. 148:2630–2634.

29. Yadav VK, Ryu JH, Suda N, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008. 135:825–837.

30. Yadav VK, Arantes HP, Barros ER, Lazaretti-Castro M, Ducy P. Genetic analysis of Lrp5 function in osteoblast progenitors. Calcif Tissue Int. 2010. 86:382–388.

31. Frost M, Andersen TE, Yadav V, Brixen K, Karsenty G, Kassem M. Patients with high-bone-mass phenotype owing to Lrp5-T253I mutation have low plasma levels of serotonin. J Bone Miner Res. 2010. 25:673–675.

32. Mödder UI, Achenbach SJ, Amin S, Riggs BL, Melton LJ 3rd, Khosla S. Relation of serum serotonin levels to bone density and structural parameters in women. J Bone Miner Res. 2010. 25:415–422.

33. Yadav VK, Oury F, Suda N, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009. 138:976–989.

34. Yadav VK, Balaji S, Suresh PS, et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med. 2010. 16:308–312.

35. Brunkow ME, Gardner JC, Van Ness J, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001. 68:577–589.

36. Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet. 2001. 10:537–543.

37. Akhter MP, Wells DJ, Short SJ, et al. Bone biomechanical properties in LRP5 mutant mice. Bone. 2004. 35:162–169.

38. Babij P, Zhao W, Small C, et al. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003. 18:960–974.

39. Ellies DL, Viviano B, McCarthy J, et al. Bone density ligand, Sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res. 2006. 21:1738–1749.

40. Zhang Y, Wang Y, Li X, et al. The LRP5 high-bone-mass G171V mutation disrupts LRP5 interaction with Mesd. Mol Cell Biol. 2004. 24:4677–4684.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download