Abstract
Monogenic disorder is a single gene disorder resulted of a single mutated gene. Monogenic disorder has benefits in early diagnosis and precious prediction of disease course. Furthermore, monogenic disorder could provide an informative knowledge to the understanding of related pathophysiology. Thyroid monogenic disorder could occur in various steps, such as thyroid development, hormonogenesis, TSH-receptor signaling, thyroid hormone transport and end organ response. Here, we reviewed of congenital hypothyroidism, congenital hyperthyroidism and thyroid hormone resistance syndrome.
Figures and Tables
Fig. 1
Monogenic disorders of thyroid disease. Genes associated with thyroid gland development, thyroid hormone synthesis, transport, action through TSH receptor or central regulation by Hypothalamus-Pituitary-Thyroid axis could be a cause of monogenic thyroid disease.

Fig. 2
Molecular genetic approaches of congenital hypothyroidism (CH).11) PDT: perchlorate discharge test, PIOD: partial iodide organification defect (PDT<90%), TIOD: total iodide organification defect (PDT>90%), NKX2.1: also known as TTF-1, TITF1, or T/EBP, FOXE1: also known as TTF-2, TITF2 or FKHL15, PAX-8: paired box transcription factor 8, TSH: thyrotropin, TSHR: receptor for TSH, NIS: sodium iodide symporter, SLC26A4: gene encoding pendrin (also known as PDS, Pendred syndrome), a multifunctional anion exchanger, DEHAL1: iodotyrosine deiodinase, TG: thyroglobulin, TPO: thyroperoxidase, DUOX2: dual oxidase 2, DUOXA2: dual oxidase maturation factor A2.
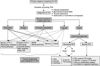
Fig. 3
Clinical characteristics of thyroid hormone resistance syndrome (RTH). Generalized thyroid receptor (TR) β mutation tends to show mental retardation with no definite abnormality of thyroid hormonal function. When the effects of TR β mutation is dominant in pituitary rather than peripheral tissues, patients show clinical hyperthyroidism. On the contrary, the effects of TR β mutation is dominant in peripheral tissues rather than pituitary, hypothyroidism is the main clinical manifestations.
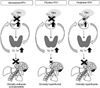
Fig. 4
Pathophysiology of congenital hypothyroidism. Congenital hypothyroidism shows diverse phenotypes according to the strength of thyroid stimulating hormone receptor (THSR), number of affected cells, and disease onset.37)

References
3. Spitzweg C, Morris JC. Genetics and phenomics of hypothyroidism and goiter due to NIS mutations. Mol Cell Endocrinol. 2010. 322(1-2):56–63.

4. Bizhanova A, Kopp P. Genetics and phenomics of Pendred syndrome. Mol Cell Endocrinol. 2010. 322(1-2):83–90.

5. Ris-Stalpers C, Bikker H. Genetics and phenomics of hypothyroidism and goiter due to TPO mutations. Mol Cell Endocrinol. 2010. 322(1-2):38–43.

6. Grasberger H. Defects of thyroidal hydrogen peroxide generation in congenital hypothyroidism. Mol Cell Endocrinol. 2010. 322(1-2):99–106.

7. Moreno JC, Visser TJ. Genetics and phenomics of hypothyroidism and goiter due to iodotyrosine deiodinase (DEHAL1) gene mutations. Mol Cell Endocrinol. 2010. 322(1-2):91–98.

8. Targovnik HM, Esperante SA, Rivolta CM. Genetics and phenomics of hypothyroidism and goiter due to thyroglobulin mutations. Mol Cell Endocrinol. 2010. 322(1-2):44–55.

9. Baas F, van Ommen GJ, Bikker H, Arnberg AC, de Vijlder JJ. The human thyroglobulin gene is over 300 kb long and contains introns of up to 64 kb. Nucleic Acids Res. 1986. 14(13):5171–5186.

10. Malthiery Y, Lissitzky S. Primary structure of human thyroglobulin deduced from the sequence of its 8448-base complementary DNA. Eur J Biochem. 1987. 165(3):491–498.

11. Targovnik HM, Citterio CE, Rivolta CM. Thyroglobulin gene mutations in congenital hypothyroidism. Horm Res Paediatr. 2011. 75(5):311–321.

12. Caron P, Moya CM, Malet D, Gutnisky VJ, Chabardes B, Rivolta CM, et al. Compound heterozygous mutations in the thyroglobulin gene (1143delC and 6725G-->A [R2223H]) resulting in fetal goitrous hypothyroidism. J Clin Endocrinol Metab. 2003. 88(8):3546–3553.

13. Pardo V, Rubio IG, Knobel M, Aguiar-Oliveira MH, Santos MM, Gomes SA, et al. Phenotypic variation among four family members with congenital hypothyroidism caused by two distinct thyroglobulin gene mutations. Thyroid. 2008. 18(7):783–786.

14. Ribault V, Castanet M, Bertrand AM, Guibourdenche J, Vuillard E, Luton D, et al. Experience with intraamniotic thyroxine treatment in nonimmune fetal goitrous hypothyroidism in 12 cases. J Clin Endocrinol Metab. 2009. 94(10):3731–3739.

15. Schwartz HL, Lazar MA, Oppenheimer JH. Widespread distribution of immunoreactive thyroid hormone beta 2 receptor (TR beta 2) in the nuclei of extrapituitary rat tissues. J Biol Chem. 1994. 269(40):24777–24782.

16. Hayashi Y, Janssen OE, Weiss RE, Murata Y, Seo H, Refetoff S. The relative expression of mutant and normal thyroid hormone receptor genes in patients with generalized resistance to thyroid hormone determined by estimation of their specific messenger ribonucleic acid products. J Clin Endocrinol Metab. 1993. 76(1):64–69.

17. Weiss RE, Weinberg M, Refetoff S. Identical mutations in unrelated families with generalized resistance to thyroid hormone occur in cytosine-guanine-rich areas of the thyroid hormone receptor beta gene. Analysis of 15 families. J Clin Invest. 1993. 91(6):2408–2415.

18. Refetoff S, Dumitrescu AM. Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract Res Clin Endocrinol Metab. 2007. 21(2):277–305.

19. Macchia PE, Takeuchi Y, Kawai T, Cua K, Gauthier K, Chassande O, et al. Increased sensitivity to thyroid hormone in mice with complete deficiency of thyroid hormone receptor alpha. Proc Natl Acad Sci U S A. 2001. 98(1):349–354.

20. Suzuki H, Willingham MC, Cheng SY. Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid. 2002. 12(11):963–969.

21. Flamant F, Samarut J. Thyroid hormone receptors: lessons from knockout and knock-in mutant mice. Trends Endocrinol Metab. 2003. 14(2):85–90.

22. Visser WE, Friesema EC, Visser TJ. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol. 2011. 25(1):1–14.

23. Heuer H, Maier MK, Iden S, Mittag J, Friesema EC, Visser TJ, et al. The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations. Endocrinology. 2005. 146(4):1701–1706.

24. Wirth EK, Roth S, Blechschmidt C, Holter SM, Becker L, Racz I, et al. Neuronal 3',3,5-triiodothyronine (T3) uptake and behavioral phenotype of mice deficient in Mct8, the neuronal T3 transporter mutated in Allan-Herndon-Dudley syndrome. J Neurosci. 2009. 29(30):9439–9449.

25. Alkemade A, Friesema EC, Kuiper GG, Wiersinga WM, Swaab DF, Visser TJ, et al. Novel neuroanatomical pathways for thyroid hormone action in the human anterior pituitary. Eur J Endocrinol. 2006. 154(3):491–500.

26. Alkemade A, Friesema EC, Unmehopa UA, Fabriek BO, Kuiper GG, Leonard JL, et al. Neuroanatomical pathways for thyroid hormone feedback in the human hypothalamus. J Clin Endocrinol Metab. 2005. 90(7):4322–4334.

27. Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004. 74(1):168–175.

28. Lafreniere RG, Carrel L, Willard HF. A novel transmembrane transporter encoded by the XPCT gene in Xq13.2. Hum Mol Genet. 1994. 3(7):1133–1139.

29. Maranduba CM, Friesema EC, Kok F, Kester MH, Jansen J, Sertie AL, et al. Decreased cellular uptake and metabolism in Allan-Herndon-Dudley syndrome (AHDS) due to a novel mutation in the MCT8 thyroid hormone transporter. J Med Genet. 2006. 43(5):457–460.

30. Friesema EC, Jansen J, Heuer H, Trajkovic M, Bauer K, Visser TJ. Mechanisms of disease: psychomotor retardation and high T3 levels caused by mutations in monocarboxylate transporter 8. Nat Clin Pract Endocrinol Metab. 2006. 2(9):512–523.

31. Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology. 2006. 147(9):4036–4043.

32. Trajkovic M, Visser TJ, Mittag J, Horn S, Lukas J, Darras VM, et al. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest. 2007. 117(3):627–635.

33. Lescure A, Allmang C, Yamada K, Carbon P, Krol A. cDNA cloning, expression pattern and RNA binding analysis of human selenocysteine insertion sequence (SECIS) binding protein 2. Gene. 2002. 291(1-2):279–285.

34. Rosenthal D. Kinetic analysis of iodine and thyroxine metabolism in "hot" thyroid nodules. Metabolism. 1981. 30(4):384–392.

35. Hebrant A, van Staveren WC, Maenhaut C, Dumont JE, Leclere J. Genetic hyperthyroidism: hyperthyroidism due to activating TSHR mutations. Eur J Endocrinol. 2011. 164(1):1–9.

36. Krohn K, Fuhrer D, Holzapfel HP, Paschke R. Clonal origin of toxic thyroid nodules with constitutively activating thyrotropin receptor mutations. J Clin Endocrinol Metab. 1998. 83(1):130–134.

37. Congdon T, Nguyen LQ, Nogueira CR, Habiby RL, Medeiros-Neto G, Kopp P. A novel mutation (Q40P) in PAX8 associated with congenital hypothyroidism and thyroid hypoplasia: evidence for phenotypic variability in mother and child. J Clin Endocrinol Metab. 2001. 86(8):3962–3967.

38. Macchia PE, Lapi P, Krude H, Pirro MT, Missero C, Chiovato L, et al. PAX8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat Genet. 1998. 19(1):83–86.

39. Krude H, Schutz B, Biebermann H, von Moers A, Schnabel D, Neitzel H, et al. Choreoathetosis, hypothyroidism, and pulmonary alterations due to human NKX2-1 haploinsufficiency. J Clin Invest. 2002. 109(4):475–480.

40. Pohlenz J, Dumitrescu A, Zundel D, Martine U, Schonberger W, Koo E, et al. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest. 2002. 109(4):469–473.

41. Clifton-Bligh RJ, Wentworth JM, Heinz P, Crisp MS, John R, Lazarus JH, et al. Mutation of the gene encoding human TTF-2 associated with thyroid agenesis, cleft palate and choanal atresia. Nat Genet. 1998. 19(4):399–401.

42. Fujiwara H, Tatsumi K, Miki K, Harada T, Miyai K, Takai S, et al. Congenital hypothyroidism caused by a mutation in the Na+/I- symporter. Nat Genet. 1997. 16(2):124–125.

43. Abramowicz MJ, Targovnik HM, Varela V, Cochaux P, Krawiec L, Pisarev MA, et al. Identification of a mutation in the coding sequence of the human thyroid peroxidase gene causing congenital goiter. J Clin Invest. 1992. 90(4):1200–1204.

44. Moreno JC, Bikker H, Kempers MJ, van Trotsenburg AS, Baas F, de Vijlder JJ, et al. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med. 2002. 347(2):95–102.

45. Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet. 1997. 17(4):411–422.

46. Ieiri T, Cochaux P, Targovnik HM, Suzuki M, Shimoda S, Perret J, et al. A 3' splice site mutation in the thyroglobulin gene responsible for congenital goiter with hypothyroidism. J Clin Invest. 1991. 88(6):1901–1905.

47. Corral J, Martin C, Perez R, Sanchez I, Mories MT, San Millan JL, et al. Thyroglobulin gene point mutation associated with non-endemic simple goitre. Lancet. 1993. 341(8843):462–464.

48. Biebermann H, Schoneberg T, Krude H, Schultz G, Gudermann T, Gruters A. Mutations of the human thyrotropin receptor gene causing thyroid hypoplasia and persistent congenital hypothyroidism. J Clin Endocrinol Metab. 1997. 82(10):3471–3480.

49. Sunthornthepvarakui T, Gottschalk ME, Hayashi Y, Refetoff S. Brief report: resistance to thyrotropin caused by mutations in the thyrotropin-receptor gene. N Engl J Med. 1995. 332(3):155–160.

50. Weinstein LS, Gejman PV, Friedman E, Kadowaki T, Collins RM, Gershon ES, et al. Mutations of the Gs alpha-subunit gene in Albright hereditary osteodystrophy detected by denaturing gradient gel electrophoresis. Proc Natl Acad Sci U S A. 1990. 87(21):8287–8290.

51. Rodien P, Bremont C, Sanson ML, Parma J, Van Sande J, Costagliola S, et al. Familial gestational hyperthyroidism caused by a mutant thyrotropin receptor hypersensitive to human chorionic gonadotropin. N Engl J Med. 1998. 339(25):1823–1826.

52. Duprez L, Parma J, Van Sande J, Allgeier A, Leclere J, Schvartz C, et al. Germline mutations in the thyrotropin receptor gene cause non-autoimmune autosomal dominant hyperthyroidism. Nat Genet. 1994. 7(3):396–401.

53. Parma J, Duprez L, Van Sande J, Cochaux P, Gervy C, Mockel J, et al. Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature. 1993. 365(6447):649–651.

54. Mastorakos G, Mitsiades NS, Doufas AG, Koutras DA. Hyperthyroidism in McCune-Albright syndrome with a review of thyroid abnormalities sixty years after the first report. Thyroid. 1997. 7(3):433–439.

55. Mori Y, Seino S, Takeda K, Flink IL, Murata Y, Bell GI, et al. A mutation causing reduced biological activity and stability of thyroxine-binding globulin probably as a result of abnormal glycosylation of the molecule. Mol Endocrinol. 1989. 3(3):575–579.

56. Mori Y, Miura Y, Takeuchi H, Igarashi Y, Sugiura J, Saito H, et al. Gene amplification as a cause of inherited thyroxine-binding globulin excess in two Japanese families. J Clin Endocrinol Metab. 1995. 80(12):3758–3762.

57. Moses AC, Rosen HN, Moller DE, Tsuzaki S, Haddow JE, Lawlor J, et al. A point mutation in transthyretin increases affinity for thyroxine and produces euthyroid hyperthyroxinemia. J Clin Invest. 1990. 86(6):2025–2033.

58. Sunthornthepvarakul T, Angkeow P, Weiss RE, Hayashi Y, Refetoff S. An identical missense mutation in the albumin gene results in familial dysalbuminemic hyperthyroxinemia in 8 unrelated families. Biochem Biophys Res Commun. 1994. 202(2):781–787.

59. Sunthornthepvarakul T, Likitmaskul S, Ngowngarmratana S, Angsusingha K, Kitvitayasak S, Scherberg NH, et al. Familial dysalbuminemic hypertriiodothyroninemia: a new, dominantly inherited albumin defect. J Clin Endocrinol Metab. 1998. 83(5):1448–1454.

60. Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004. 364(9443):1435–1437.

61. Weiss RE, Refetoff S. Resistance to thyroid hormone. Rev Endocr Metab Disord. 2000. 1(1-2):97–108.
62. Bjorses P, Aaltonen J, Horelli-Kuitunen N, Yaspo ML, Peltonen L. Gene defect behind APECED: a new clue to autoimmunity. Hum Mol Genet. 1998. 7(10):1547–1553.

63. Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002. 39(8):537–545.

64. Friedman DL, Kastner T, Pond WS, O'Brien DR. Thyroid dysfunction in individuals with Down syndrome. Arch Intern Med. 1989. 149(9):1990–1993.

65. Karlsson B, Gustafsson J, Hedov G, Ivarsson SA, Anneren G. Thyroid dysfunction in Down's syndrome: relation to age and thyroid autoimmunity. Arch Dis Child. 1998. 79(3):242–245.

66. Murdoch JC, Ratcliffe WA, McLarty DG, Rodger JC, Ratcliffe JG. Thyroid function in adults with Down's syndrome. J Clin Endocrinol Metab. 1977. 44(3):453–458.

67. Adachi M, Tachibana K, Masuno M, Makita Y, Maesaka H, Okada T, et al. Clinical characteristics of children with hypoparathyroidism due to 22q11.2 microdeletion. Eur J Pediatr. 1998. 157(1):34–38.

68. Kawame H, Adachi M, Tachibana K, Kurosawa K, Ito F, Gleason MM, et al. Graves' disease in patients with 22q11.2 deletion. J Pediatr. 2001. 139(6):892–895.

69. Kawamura T, Nimura I, Hanafusa M, Fujikawa R, Okubo M, Egusa G, et al. DiGeorge syndrome with Graves' disease: a case report. Endocr J. 2000. 47(1):91–95.

70. Elsheikh M, Wass JA, Conway GS. Autoimmune thyroid syndrome in women with Turner's syndrome-the association with karyotype. Clin Endocrinol (Oxf). 2001. 55(2):223–226.

71. Medeiros CC, Marini SH, Baptista MT, Guerra G Jr, Maciel-Guerra AT. Turner's syndrome and thyroid disease: a transverse study of pediatric patients in Brazil. J Pediatr Endocrinol Metab. 2000. 13(4):357–362.

72. Radetti G, Mazzanti L, Paganini C, Bernasconi S, Russo G, Rigon F, et al. Frequency, clinical and laboratory features of thyroiditis in girls with Turner's syndrome The Italian Study Group for Turner's Syndrome. Acta Paediatr. 1995. 84(8):909–912.

73. Greenberg F, Lewis RA, Potocki L, Glaze D, Parke J, Killian J, et al. Multi-disciplinary clinical study of Smith-Magenis syndrome (deletion 17p11.2). Am J Med Genet. 1996. 62(3):247–254.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download