Abstract
Objectives
The purpose of this study was to determine the setting time, compressive strength, solubility, and pH of mineral trioxide aggregate (MTA) mixed with glass ionomer cement (GIC) and to compare these properties with those of MTA, GIC, IRM, and SuperEBA.
Materials and Methods
Setting time, compressive strength, and solubility were determined according to the ISO 9917 or 6876 method. The pH of the test materials was determined using a pH meter with specified electrode for solid specimen.
Results
The setting time of MTA mixed with GIC was significantly shorter than that of MTA. Compressive strength of MTA mixed with GIC was significantly lower than that of other materials at all time points for 7 days. Solubility of 1 : 1 and 2 : 1 specimen from MTA mixed with GIC was significantly higher than that of other materials. Solubility of 1 : 2 specimen was similar to that of MTA. The pH of MTA mixed with GIC was 2-4 immediately after mixing and increased to 5-7 after 1 day.
Conclusions
The setting time of MTA mixed with GIC was improved compared with MTA. However, other properties such as compressive strength and pH proved to be inferior to those of MTA. To be clinically feasible, further investigation is necessary to find the proper mixing ratio in order to improve the drawbacks of MTA without impairing the pre-existing advantages and to assess the biocompatibility.
Mineral trioxide aggregate (MTA) is widely used in endodontic therapy. It has been used as pulp-capping material, root-end filling material and perforation-repair material.1
MTA is a powder consisting of fine hydrophilic particles of tricalcium silicate, tricalcium aluminate, tricalcium oxide, silicate oxide and other mineral oxides, which set in the presence of moisture.1,2 MTA has proven to be a biocompatible material in numerous studies.3-7 MTA has been shown to have the least cytotoxicity compared to other materials and to promote cementum formation and apical root closures.8 MTA was also found to be the most effective material in preventing leakage9 as a perforation repair material and a root-end filling material.10
Although MTA has many favorable properties, there are several drawbacks. The setting time of MTA has been reported to be about 3 hours,11 which may impair the integrity of MTA during the setting period. Another drawback is its difficult handling characteristics. The mixture of MTA and distilled water is difficult to deliver to the required site and hard to compact adequately.10 Moreover, the cost of MTA is also relatively high.12
Glass ionomer cements are formed by the reaction of calcium-aluminosilicate glass particles with aqueous solutions of polyacrylic acid. It bonds physicochemically to dentine. Biocompatibility studies have shown evidence of initial cytotoxicity with freshly prepared samples, with decreasing toxicity as setting occurs. It is easy to handle and does not cause any adverse histological reaction in the periapical tissue.13
The desire to change some properties of MTA, especially handling and the setting time, has been discussed. 14,15 The use of setting accelerators such as calcium chloride (CaCl2) and sodium phosphate dibasic (Na2HPO4) and its effect on the properties of MTA have been evaluated.16,17 Researches on using another vehicle such as propylenglycol, articaine solution, and chlorhexidine rather than water or saline have been also reported.15,18,19 Because GIC has been also used for perforation repair or root end filling and widely known for its biocompatibility,20,21 it would be of interest to investigate the feasibility of using MTA mixed with GIC.
The purpose of this study was to determine the setting time, compressive strength, solubility, and pH of MTA mixed with GIC and to compare these properties with those of MTA, GIC, IRM, and SuperEBA. This study was designed with the intention of improving the handling characteristics of MTA and its ultimate purpose was to evaluate the clinical feasibility of MTA mixed with GIC.
Four materials were used: white mineral trioxide aggregate (MTA: ProRoot MTA, Dentsply Tulsa Dental, Tulsa, OK, USA), glass ionomer cement (GIC: Fuji II, GC Corporation, Tokyo, Japan), two types of reinforced zinc oxide-eugenol cements (IRM, Dentsply International Inc., York, PA, USA and SuperEBA fast set, Bosworth Company, Skokie, IL, USA).
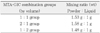
Experimental groups were divided into the following 3 categories depending on the MTA and GIC powder mixture ratios by volume: 1) 1 : 1 group (MTA powder : GIC powder = 1 : 1) 2) 2 : 1 group (MTA powder : GIC powder = 2 : 1) 3) 1 : 2 group (MTA powder : GIC powder = 1 : 2). The experimental powder was mixed with GIC liquid instead of distilled water. Powder-to-liquid mixing ratios for MTA-GIC combination groups are described in Table 1. Powder-to-liquid ratio for each MTA-GIC combination group was the same as each other in volume because the same level scoop and liquid dispensing method were used. However, it varied in weight because the same volume of MTA weighs more than that of GIC powder.
All the other groups except the MTA-GIC combination groups were mixed according to the manufacturers' instructions.
The setting times of test materials were determined according to the ISO 9917 method 22,23 with a Vicat apparatus. The Vicat indenter is 400 ± 5 g in weight with a needle having a flat end of 1.0 ± 0.1 mm in diameter.
Each material was mixed and placed in a circular acrylic mold (10 mm of inner diameter and 5 mm of height). The assembly was placed in a cabinet at 37℃ and relative humidity of 95%. Ninety seconds after the end of mixing, the indenter needle was carefully lowered vertically on to the surface of the material and allowed to remain there for 5 seconds. To determine the approximate setting time, the indentations were repeated at 30 seconds intervals until the needle fails to make a complete circular indentation in the test material. This process was repeated starting the indentation at 30 seconds before the approximate setting time thus determined, making indentations at 10 seconds intervals. This test was repeated 10 times for each material.
The compressive strengths of the test materials were determined by the method of the ISO 9917.22 Each material was mixed and placed in split stainless steel molds (internal dimensions 6.0 ± 0.1 mm high and 4.0 ± 0.1 mm of internal diameter).
No later than 120 seconds after the end of mixing, the whole assembly was transferred to the cabinet maintained at 37℃ for 6 hours. The specimens were removed from the molds and checked visually for air-voids or chipped edges. Any such defective specimens were discarded. The specimens were immersed in distilled water for 24 hours, 3 days, and 7 days and maintained at 37℃. Then their compressive strengths were measured using an universal testing machine (Instron, Model GB/4302, Instron Corp., High Wycombe, UK) at the crosshead speed of 1.0 mm/min. The maximum load required to fracture each specimen was measured and the compressive strength (C) was calculated in megapascals according to the formula.
C = 4P / πD2
where P is the maximum load applied in Newton and D is the mean diameter of the specimen in millimeters. This test was repeated 10 times for each material.
The solubility of the test materials was assessed in accordance with the ISO 6876 standard.24,25 Each material was mixed and placed in two split-ring molds (internal diameter 20 ± 1 mm, 1.5 ± 0.1 mm high). The filled molds were placed in the cabinet maintained at 37℃ and a relative humidity of 95% for a period of time 50% longer than the setting time. The specimens were removed from the molds and the mass of the each specimen was determined to the nearest 0.001 g with the precision scale (A200S, Sartorius, Goettingen, Germany; precision = 0.0001 g). Two such specimens were placed in the shallow dish and 50 ± 1 mL of distilled water was added. The dish was covered and placed in the cabinet for 24 hours. The specimens were removed and washed with 2-3 mL of fresh water, recovering the washings in the shallow dish. The water was then evaporated from the dish without boiling and dried to constant mass at 110 ± 2℃. After cooled, the dish was weighed. The differences found between this weight and the original dish weight were divided into the initial dry weight of the specimens and multiplied by 100. The result was recorded as solubility. This test was repeated 6 times for each material.
The test material was mixed and placed in circular acrylic mold (10 mm of inner diameter, 5 mm of height). The pH was measured with a pH meter (Delta350, Mettler Toledo, Schwerzenbach, Switzerland) using an electrode for solid specimen (InLab Surface, Mettler Toledo, Schwerzenbach, Switzerland) at the end of mixing, after 10 minutes, 6 hours, and 24 hours. This test was repeated 6 times for each material.
The apparatus was previously calibrated with pH 7.0 and pH 4.0 solutions. Between each measurement the electrode was washed with distilled water and blot dried.
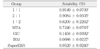
The setting times of the test materials are listed in Table 2. The setting time of MTA mixed with GIC was significantly shorter than that of MTA (p < 0.01). Especially the setting time of 1 : 1 and 2 : 1 group specimen was similar to that of GIC, IRM, and SuperEBA.
SuperEBA, 2 : 1 group, 1 : 1 group, GIC, and IRM set significantly faster than 1 : 2 group and MTA (p < 0.01). The setting time of MTA was significantly longer than that of all the other groups (p < 0.01).
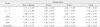
The means and standard deviations of compressive strength (MPa) of the test materials are shown in Table 3. The compressive strength of all materials increased during 1 week. As for MTA mixed with GIC, there was no significant difference between the time points. On the contrary, compressive strength of MTA, GIC, IRM, and SuperEBA increased significantly between 1 day and 3 days (p < 0.01).
Compressive strength of MTA mixed with GIC was significantly lower than that of other materials at all time points for 7 days (p < 0.05). Especially after 3 days and 7 days, the difference was statistically significant at the p value of less than 0.01.
The results of solubility are listed in Table 4. Solubility of 1 : 1 and 2 : 1 specimens was significantly higher than that of other materials (p < 0.05). Solubility of 1 : 2 specimen was similar to that of MTA and was significantly higher than that of GIC, IRM, and SuperEBA (p < 0.05).
The results of pH are listed in Table 5. The pH of MTA mixed with GIC was 2-4 immediately after mixing and increased to 5-7 after 1 day. The differences between each time point were significant (p < 0.05). After 6 hours and 24 hours, pH of MTA mixed with GIC was significantly lower than that of MTA and GIC (p < 0.01).
Overall setting phases of MTA mixed with distilled water have been documented in previous studies.26,27 The setting of MTA occurs in three stages. In the first 24 hours, the tricalcium aluminate hydrates to form hydrated colloidal gel of tricalcium aluminate. The second phase occurs between the 1st and 7th days. Tricalcium silicates and tricalcium aluminate react with water to form Ca(OH)2, aluminum hydroxide, and amorphous calcium silicate. The third phase of cement setting is a slow reaction and occurs between the 7th and 28th days. During that period the calcium silicates progressively hydrate to form hydrated silicate gel, and Ca(OH)2 becomes studded in this gel, imparting strength to the set cement.
Setting reactions of GIC is well known and analyzed.28 The acidic liquid solution dissolves portions of the periphery of the silicate glass particle, releasing calcium, aluminum, and other ions. Calcium ions are chelated by carboxyl groups on polyacrylic acid, producing an amorphous polymer gel. During the next 24 to 72 hours, the calcium ions are replaced by more slowly reaction aluminum ions to produce a more highly cross-linked matrix.
In this study the setting time of MTA mixed with GIC was significantly shorter than that of MTA (p < 0.05). Its fast set was possibly caused by GIC's acid base reaction. However, the reaction of MTA powder with GIC liquid is not clear and whether the MTA powder was completely set or not has not been precisely proven. In clinical situations, MTA powder may react with the surrounding tissue fluids. However it could be possible that much of MTA powder was entrapped in the GIC's matrix. Based on the previous report,27 two phenomena are observed in the spectral analysis of the setting of MTA: decrease of SiO2 and increase of Ca(OH)2. For further studies, spectroscopic analysis may be required to assess the setting reaction of MTA mixed with GIC.
In Table 3, the setting time of 1 : 2 group was significantly longer than that of 1 : 1 and 2 : 1 groups. This may relate to the fact that powder-to-liquid ratio for this group is too small. In this study, the same powder-to-liquid ratio by volume was used for all MTA-GIC combination groups in order to minimize the effect of GIC liquid between groups. However, even with smaller amount of GIC liquid, 1 : 2 group was mixed properly and set sooner in the preliminary study. Therefore, it is advisable to use the individual optimal powder-to-liquid ratio for each MTA-GIC combination group in further study.
According to the ISO 9917 method on determining compressive strength, the specimen is supposed to be removed from the mold 1 hour after mixing,24 but in this study this was performed after 6 hours because MTA sets after 3-4 hours.
In the present study, MTA mixed with GIC had the lowest compressive strength among materials tested. Although those values increased with time, they were significantly lower than those of other materials at all time points (p < 0.05). It may have some relation to the uncertain setting reaction of MTA mixed with GIC. Because root-end filling materials do not bear direct pressure, the compressive strength of these materials is not as important as those materials used to repair defects in occlusal surfaces.11 However, compressive strength is important for stability of the restored site and there is still room for improvement by adjusting the powder-to-liquid ratio.
Solubility of MTA mixed with GIC was greater than that of other materials. Because solubility was determined only after first 24 hours in this study, short period could have some relation to the result. To be clinically more meaningful, longer test period and analysis of dissolved elements are necessary. Solubility of MTA after 24 hours was in agreement with the previous report.29
There are some studies on pH which determined the pH of extracts after the test materials were immersed in distilled water.16,30,31 However there were some disadvantages. The results were variable depending on the amount of the distilled water and the immersion period. Another disadvantage is that test materials were immersed while contained in tube and the diameter of tube also affected the results. Because it was not the pH of the material itself but the pH of the solved constituents, its meaning could be limited. In this study pH was measured by the direct contact of the pH electrode and the mixed material and the electrode was designed for the surface contact. The result that the pH of MTA was 12.15 at 6 hours after mixing, is in agreement with the previous report by Torabinejad et al.11
The powder-to-liquid ratios used in the MTA mixed with GIC group are varying in weight (Table 1). However as mentioned before, the ratios in volume are the same between the 3 groups because the scoops of same volume were used in this experiment in order to minimize the effect of amount of GIC liquid on the results of the experiment. If the powder-to-liquid ratios used in the MTA mixed with GIC group are adjusted individually depending on the mixture ratios of MTA powder and GIC powder, many properties including compressive strength and solubility may be improved.
One of the drawbacks of MTA is its handling characteristics. Unlike GIC, IRM, and SuperEBA, MTA is not easy to deliver and to compact. On the contrary, handling characteristics of MTA mixed with GIC was improved compared to MTA. MTA mixed with GIC was generally easier to deliver to the required site and to compact adequately than MTA.
Two modifications of zinc oxide-eugenol cements, IRM and SuperEBA have been shown to have good sealing ability and high compressive strength.11 In this study, they presented high compressive strength and low solubility. However there are potential disadvantages of using zinc oxide-eugenol cements as retrograde root fillings because of their unreacted eugenol.32
The results from this study can be summarized as follows.
The setting time of MTA mixed with GIC was significantly shorter than that of MTA (p < 0.01). Especially the setting time of 1 : 1 and 2 : 1 specimen was similar to that of GIC, IRM, and SuperEBA.
Compressive strength of MTA mixed with GIC was significantly lower than that of other materials at all time points for 7 days (p < 0.05) and there was no significant difference between time points. On the contrary, compressive strength of MTA, GIC, IRM, and SuperEBA increased significantly between 1 day and 3 days (p < 0.01).
Solubility of 1 : 1 and 2 : 1 specimen from MTA mixed with GIC was significantly higher than that of other materials (p < 0.05). Solubility of 1 : 2 specimen was similar to that of MTA and was significantly higher than that of GIC, IRM, and SuperEBA (p < 0.05).
The pH of MTA mixed with GIC was 2-4 immediately after mixing and increased to 5-7 after 1 day. The differences between each time point were significant (p < 0.05). After 6 hours and 24 hours, pH of MTA mixed with GIC was significantly lower than that of MTA and GIC (p < 0.01).
Based on the results above, the setting time of MTA mixed with GIC was improved compared with MTA. However, some properties of MTA mixed with GIC like compressive strength, proved to be inferior to those of MTA. To be clinically feasible, further investigations are necessary to find the proper mixing ratio in order to improve the drawbacks of MTA without impairing the pre-existing advantages and to assess the biocompatibility.
Figures and Tables
References
1. Bodrumlu E. Biocompatibility of retrograde root filling materials: a review. Aust Endod J. 2008. 34:30–35.

2. Chang SW, Yoo HM, Park DS, Oh TS, Bae KS. Ingredients and cytotoxicity of MTA and 3 kinds of Portland cements. J Korean Acad Conserv Dent. 2008. 33:369–376.

3. Torabinejad M, Hong CU, Pitt Ford TR, Kettering JD. Cytotoxicity of four root end filling materials. J Endod. 1995. 21:489–492.

4. Huang TH, Yang CC, Ding SJ, Yan M, Chou MY, Kao CT. Biocompatibility of human osteosarcoma cells to root end filling materials. J Biomed Mater Res B Appl Biomater. 2005. 72:140–145.

5. Kang MK, Bae IH, Koh JT, Hwang YC, Hwang IN, Oh WM. Comparison of Biocompatibility of Four Root perforation repair Materials. J Korean Acad Conserv Dent. 2009. 34:192–198.

6. Koh ET, McDonald F, Pitt Ford TR, Torabinejad M. Cellular response to Mineral Trioxide Aggregate. J Endod. 1998. 24:543–547.

7. Yun YR, Yang IS, Hwang YC, Hwang IN, Choi HR, Yoon SJ, Kim SH, Oh WM. Pulp response of Mineral trioxide aggregate, calcium sulfate or calcium hydroxide. J Korean Acad Conserv Dent. 2007. 32:95–101.

8. Torabinejad M, Pitt Ford TR, McKendry DJ, Abedi HR, Miller DA, Kariyawasam SP. Histologic assessment of mineral trioxide aggregate as a root-end filling in monkeys. J Endod. 1997. 23:225–228.

9. Hashem AA, Hassanien EE. ProRoot MTA, MTA-Angelus and IRM used to repair large furcation perforations: sealability study. J Endod. 2008. 34:59–61.

10. Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of MTA. J Endod. 2006. 32:569–572.

11. Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995. 21:349–353.

12. Bortoluzzi EA, Broon NJ, Bramante CM, Felippe WT, Tanomaru Filho M, Esberard RM. The influence of calcium chloride on the setting time, solubility, disintegration, and pH of mineral trioxide aggregate and white Portland cement with a radiopacifier. J Endod. 2009. 35:550–554.

13. Vasudev SK, Goel BR, Tyagi S. Root end filling materials - a review. Endodontology. 2003. 15:12–18.
14. Wiltbank KB, Schwartz SA, Schindler WG. Effect of selected accelerants on the physical properties of mineral trioxide aggregate and Portland cement. J Endod. 2007. 33:1235–1238.

15. Gandolfi MG, Perut F, Ciapetti G, Mongiorgi R, Prati C. New Portland cement-based materials for endodontics mixed with articaine solution: a study of cellular response. J Endod. 2008. 34:39–44.

16. Antunes Bortoluzzi E, Juárez Broon N, Antonio Hungaro, de Oliveira Oliveira AC, Monteiro Bramante C. The use of a setting accelerator and its effect on pH and calcium ion release of mineral trioxide aggregate and white Portland cement. J Endod. 2006. 32:1194–1197.

17. Huang TH, Shie MY, Kao CT, Ding SJ. The effect of setting accelerator on properties of mineral trioxide aggregate. J Endod. 2008. 34:590–593.

18. Holland R, Mazuqueli L, de Souza V, Murata SS, Dezan Júnior E, Suzuki P. Influence of the type of vehicle and limit of obturation on apical and periapical tissue response in dogs' teeth after root canal filling with mineral trioxide aggregate. J Endod. 2007. 33:693–697.

19. Karimjee CK, Koka S, Rallis DM, Gound TG. Cellular toxicity of mineral trioxide aggregate mixed with an alternative delivery vehicle. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006. 102:e115–e120.

20. De Bruyne MA, De Moor RJ. The use of glass ionomer cements in both conventional and surgical endodontics. Int Endod J. 2004. 37:91–104.

21. de Souza Costa CA, Hebling J, Garcia-Godoy F, Hanks CT. In vitro cytotoxicity of glass-ionomer cements. Biomaterials. 2003. 24:3853–3858.
22. International Organization for Standardization. Dentistry - Water-based cements - Part 1: Powder/liquid acid-base cements. ISO 9917-1. 2007.
23. Ber BS, Hatton JF, Stewart GP. Chemical modification of ProRoot MTA to improve handling characteristics and decrease setting time. J Endod. 2007. 33:1231–1234.

24. International Organization for Standardization. Dental root canal sealing materials. ISO 6876. 2001.
25. Chng HK, Islam I, Yap AU, Tong YW, Koh ET. Properties of a new root-end filling material. J Endod. 2005. 31:665–668.

26. Dammaschke T, Gerth HU, Züchner H, Schäfer E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mater. 2005. 21:731–738.

27. Nandini S, Ballal S, Kandaswamy D. Influence of glass-ionomer cement on the interface and setting reaction of mineral trioxide aggregate when used as a furcal repair material using laser Raman spectroscopic analysis. J Endod. 2007. 33:167–172.

28. Roberson TM, Heymann HO, Swift EJ. Sturdevant's Art and Science of Operative Dentistry. 2005. Fifth Edition. Mosby Elsevier;215–220.
29. Poggio C, Lombardini M, Alessandro C, Simonetta R. Solubility of root-end-filling materials: a comparative study. J Endod. 2007. 33:1094–1097.

30. Duarte MA, Demarchi AC, Yamashita JC, Kuga MC, Fraga Sde C. pH and calcium ion release of 2 root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003. 95:345–347.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download