Abstract
The objective of this study was to observe the histology of dental pulp healing after tooth replantation in rats. The maxillary right first molars of 4-week-old rat were extracted, and then the teeth were repositioned in the original socket. At 3 days after replantation, there was localized inflammatory reaction. But, pulp revasculization and healing had already begun in the root area. At 5 days after replantation, odontoblast-like cells were observed. Tertiary dentin deposition was observed beneath the pulp-dentin border from 1 week after replantation. And tertiary dentin was increased at 2 weeks after replantation. The presence of odontoblast-like cells and the formation of tertiary dentin were continued to 4 weeks after replantation. At 4 weeks after replantation, the deposition of bone-like tissues and cementum-like tissues was observed. This results show that there is a possibility of pulp healing after tooth replantation in rats and the mineralization of tooth can progress. The mineralization of tooth after replantation was initially occurred by the deposition of tertiary dentin, but as time passed, the deposition of bone-like tissues and cementum-like tissues was begun and increased.
Figures and Tables
Figure 1
Control tooth. A: coronal zone (magnification, ×50). B: Higher magnification of boxed area in A. It presents normal dentinogenesis process. Odontoblasts are arranged along the pulp-dentin border. Beneath the odontoblast layer, regular tubular dentin is observed (magnification, ×400). Abbreviations: D, dentin; DP, dental pulp; OB, odontoblasts.

Figure 2
3 days after replantation. A: There is localizaed inflammation in the coronal pulp (magnification, ×50). B: Higher magnification of upper boxed area in A. Neutrophils and red blood cells are observed in the pulp horn area (magnification, ×100). C: Higher magnification of lower boxed area in A. Blood supply is recovered in the root pulp (magnification, ×200). Abbreviations: D, dentin; DP, dental pulp.

Figure 3
5 days after replantation. A: Coronal zone. Localized inflammation still remains including neutrophils and fibrin networks in the pulp horn area (magnification, ×100). B: Root zone. Formation of odontoblast-like cell layer begins at the pulp-dentin border (magnification, ×100). Abbreviations: D, dentin; DP, dental pulp; OB, odontoblast-like cells.

Figure 4
1 week after replantation. A: Inflammatory lesion disappeared in all of the pulp (magnification, ×400). B: Blood supply is recovered to the coronal pulp. No odontoblast layer formation was formed at the pulp horn area (magnification, ×100). C: At root apex area, there is formation of mineralized region which is similar to the cellular cementum tissues (arrows) (magnification, ×100). D: Higher magnification of boxed area in A. Under the pulp-dentin border, there is a layer of odontoblast-like cells. Some tertiary dentin is deposited beneath the odontoblast-like cells layer (magnification, ×200). Abbreviations: D, dentin; DP, dental pulp; OB, odontoblast-like cells; TD, tertiary dentin.
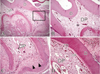
Figure 5
2 weeks after replantation. A: Tertiary dentin deposition is clear with increased thickness beneath the preexisting dentin (magnification, ×50). B: Coronal zone. Formation of odontoblast-like cells layer is discovered beneath all coronal pulp-dentin border (magnification, ×100). C: Higher magnification of right boxed area in A. At root apex area, there is increased formation of mineralized tissue. It is mixed with tertiary dentins and cementum-like tissues (arrow) (magnification, ×100). D: Higher magnification of left boxed area in A. Beneath the pulp-dentin border, there is a layer of odontoblast-like cells. Increased tertiary dentin deposition is discovered (magnification, ×400). Abbreviations: D, dentin; DP, dental pulp; OB, odontoblast-like cells; TD, tertiary dentin.
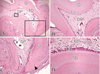
Figure 6
4 weeks after replantation. A: Tertiary dentin thickness is increased than that of 2 week after replantation. And pulp space is more narrowed (magnification, ×50). B: Mixed form of tertiary dentin and cellular cementum-like tissues is observed under the external root resorption area (arrow) (magnification, ×50). Abbreviations: D, dentin; DP, dental pulp; OB, odontoblast-like cells; TD, tertiary dentin.

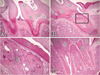
Figure 7
4 weeks after replantation. A, B: Both tertiary dentin and bone-like tissues are formed. A: Bone-like tissues are mixed with tertiary dentin. B: Bone-like tissues occupy the pulp space without tertiary dentin. (magnification, ×50). C: Higher magnification of boxed area in B. In the bone-like tissues matrix, there are Haversian canals and osteoblast-like cells (magnification, ×200). D: As a result of pulp healing, there is formation of bone-like tissue only without tertiary dentin (magnification, ×50). Abbreviations: B, bone-like tissues; C, cementum-like tissues; D, dentin; DP, dental pulp; OB, odontoblast-like cells; TD, tertiary dentin.
References
1. Jang HT, Lee HI, Lee KW, Lee SJ. Temperature changes in the pulp according to various esthetic restorative materials and bases during curing procedure. J Korean Acad Conserv Dent. 2001. 26:393–398.
2. Bae JH, Kim YG, Yoon PY, Cho BH, Choi YH. Pulp response of beagle dog to direct pulp capping materials: Histological study. J Korean Acad Conserv Dent. 2010. 35:5–12.

3. About I, Bottero MJ, de Denato P, Camps J, Franquin JC, Mitsiadis TA. Human dentin production in vitro. Exp Cell Res. 2000. 258(1):33–41.
4. Chiego DJ Jr. An ultrastructural and autoradiographic analysis of primary and replacement odontoblasts following cavity preparation and wound healing in the rat molar. Proc Finn Dent Soc. 1992. 88:Suppl 1. 243–256.
5. Goldberg M, Farges JC, Lacerda-Pinheiro S, Six N, Jegat N, Decup F, et al. Inflammatory and immunological aspects of dental pulp repair. Pharmacol Res. 2008. 58(2):137–147.

6. Harada M, Kenmotsu S, Nakasone N, Nakakura-Ohshima K, Ohshima H. Cell dynamics in the pulpal healing process following cavity preparation in rat molars. Histochem Cell Biol. 2008. 130(4):773–783.

7. Hirata M, Yamaza T, Mei YF, Akamine A. Expression of osteocalcin and Jun D in the early period during reactionary dentin formation after tooth preparation in rat molars. Cell Tissue Res. 2005. 319(3):455–465.

8. Kuratate M, Yoshiba K, Shigetani Y, Yoshiba N, Ohshima H, Okiji T. Immunohistochemical analysis of nestin, osteopontin, and proliferating cells in the reparative process of exposed dental pulp capped with mineral trioxide aggregate. J Endod. 2008. 34(8):970–974.

9. Ohshima H, Nakakura-Ohshima K, Yamamoto H, Takeyasu . Responses of odontoblasts to cavity preparation in rat molars as demonstrated by immunocytochemistry for heat shock protein (Hsp) 25. Arch Histol Cytol. 2001. 64(5):493–501.

10. Linde A. Dentin mineralization and the role of odontoblasts in calcium transport. Connect Tissue Res. 1995. 33(1-3):163–170.

11. Smith AJ. Dentin formation and repair. Seltzer and Bender's dental pulp. 2002. 41–62.
12. Stanley HR, Ranney RR. Age changes in the human dental pulp. I. The quantity of collagen. Oral Surg Oral Med Oral Pathol. 1962. 15:1396–1404.
13. Hasegawa T, Suzuki H, Yoshie H, Ohshima H. Influence of extended operation time and of occlusal force on determination of pulpal healing pattern in replanted mouse molars. Cell Tissue Res. 2007. 329(2):259–272.

14. Hosoya A, Nakamura H, Ninomiya T, Hoshi K, Yoshiba K, Yoshiba N, et al. Hard tissue formation in subcutaneously transplanted rat dental pulp. J Dent Res. 2007. 86(5):469–474.

15. Hosoya A, Yoshiba K, Yoshiba N, Hoshi K, Iwaku M, Ozawa H. An immunohistochemical study on hard tissue formation in a subcutaneously transplanted rat molar. Histochem Cell Biol. 2003. 119(1):27–35.

16. Nakakura-Ohshima K, Watanabe J, Kenmotsu S, Ohshima H. Possible role of immunocompetent cells and the expression of heat shock protein-25 in the process of pulpal regeneration after tooth injury in rat molars. J Electron Microsc (Tokyo). 2003. 52(6):581–591.

17. Ogawa R, Saito C, Jung HS, Ohshima H. Capacity of dental pulp differentiation after tooth transplantation. Cell Tissue Res. 2006. 326(3):715–724.

18. Ohshima H, Nakakura-Ohshima K, Yamamoto H, Maeda T. Alteration in the expression of heat shock protein (Hsp) 25-immunoreactivity in the dental pulp of rat molars following tooth replantation. Arch Histol Cytol. 2001. 64(4):425–437.

19. Shimizu A, Nakakura-Ohshima K, Noda T, Maeda T, Ohshima H. Responses of immunocompetent cells in the dental pulp to replantation during the regeneration process in rat molars. Cell Tissue Res. 2000. 302(2):221–233.

20. Takamori Y, Suzuki H, Nakakura-Ohshima K, Cai J, Cho SW, Jung HS, et al. Capacity of dental pulp differentiation in mouse molars as demonstrated by allogenic tooth transplantation. J Histochem Cytochem. 2008. 56(12):1075–1086.

21. Unno H, Suzuki H, Nakakura-Ohshima K, Jung HS, Ohshima H. Pulpal regeneration following allogenic tooth transplantation into mouse maxilla. Anat Rec (Hoboken). 2009. 292(4):570–579.

22. Andreasen JO, Borum MK, Jacobsen HL, Andreasen FM. Replantation of 400 avulsed permanent incisors. 2. Factors related to pulpal healing. Endod Dent Traumatol. 1995. 11(2):59–68.

23. Byers MR, Kvinnsland I, Bothwell M. Analysis of low affinity nerve growth factor receptor during pulpal healing and regeneration of myelinated and unmyelinated axons in replanted teeth. J Comp Neurol. 1992. 326(3):470–484.

24. Kvinnsland I, Heyeraas KJ, Byers MR. Regeneration of calcitonin gene-related peptide immunoreactive nerves in replanted rat molars and their supporting tissues. Arch Oral Biol. 1991. 36(11):815–826.

25. Tsukamoto-Tanaka H, Ikegame M, Takagi R, Harada H, Ohshima H. Histochemical and immunocytochemical study of hard tissue formation in dental pulp during the healing process in rat molars after tooth replantation. Cell Tissue Res. 2006. 325(2):219–229.

26. Hargreaves KM, Giesler T, Henry M, Wang Y. Regeneration potential of the young permanent tooth: what does the future hold. J Endod. 2008. 34:7 Suppl. S51–S56.

27. Chueh LH, Huang GT. Immature teeth with periradicular periodontitis or abscess undergoing apexogenesis: a paradigm shift. J Endod. 2006. 32(12):1205–1213.

28. Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004. 30(4):196–200.

29. Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001. 17(4):185–187.

30. Jung IY, Lee SJ, Hargreaves KM. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. J Endod. 2008. 34(7):876–887.

31. Thibodeau B, Trope M. Pulp revascularization of a necrotic infected immature permanent tooth: case report and review of the literature. Pediatr Dent. 2007. 29(1):47–50.
32. Kwon OT, Kum KY, Lee SJ, The effect. The effect of periodontal regeneration and anti-resorption of dexamathasone and OP-1 following delayed replantation in rat model. J Korean Acad Conserv Dent. 2001. 26:296–306.
33. Okiji T. Pulp as a connective tissue. Seltzer and Bender's dental pulp. 2002. 95–117.
34. Smith AJ, Cassidy N, Perry H, Begue-Kirn C, Ruch JV, Lesot H. Reactionary dentinogenesis. Int J Dev Biol. 1995. 39(1):273–280.
35. Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002. 81(8):531–535.

36. Batouli S, Miura M, Brahim J, Tsutsui TW, Fisher LW, Gronthos S, et al. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res. 2003. 82(12):976–981.

37. Kristerson L, Andreasen JO. Influence of root development on periodontal and pulpal healing after replantation of incisors in monkeys. Int J Oral Surg. 1984. 13(4):313–323.

38. Egelberg J. Regeneration and repair of periodontal tissues. J Periodontal Res. 1987. 22(3):233–242.

39. Wang X, Thibodeau B, Trope M, Lin LM, Huang GTJ. Histologic Characterization of Regenerated Tissues in Canal Space after the Revitalization/Revascularization Procedure of Immature Dog Teeth with Apical Periodontitis. J Endod. 2010. 36(1):56–63.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download