Abstract
The purpose of this study was to compare mineral trioxide aggregate (MTA; Dentsply, Tulsa Dental, Tulsa, OK, USA), which is widely used as root-end filling material, with DiaRoot BioAggregate (DB; Innovative BioCaramix Inc, Vancouver, BC, Canada), newly developed product, by using MG63 osteoblast-like cells. MTA, DB, and Intermediate Restorative Material (IRM; Dentsply Caulk, Milford, DE, USA) were used for root-end filling material while tissue culture plastic was used for control group. Each material was mixed and, the mixtures were left to set for 24 hours. MG63 cells were seeded to each group and then they were cultured for attachment for 4 hours. Following the attachment of cells to the root-end filling material, early cellular response was observed. After another 12 hours'culture, the level of attachment between cells and material was observed and in order to identify the effect of each material to bone formation, transforming growth factor beta1 (TGFβ1) and osteocalin (OC) were estimated by using enzyme-linked immunosorbent assay (ELISA), and the amount of alkaline phosphatase (ALP) was also measured. The data were analyzed using one-way ANOVA. As a result, only at OC and the number of cells which were attached to materials, there was no statistical difference between MTA and DB. At other items, there was statistically significant difference in all groups. Although DB has not shown exactly the same cellular response like that of MTA, the number of attached cells shows that biocompatibility of the material and OC indicates bone formation rate. Therefore, if DB is used for root end filling material, it is expected to lead to similar results to MTA.
Nowadays, in endodontic field mineral trioxide aggregate (MTA; Dentsply, Tulsa Dental, Tulsa, OK, USA) has been introduced and widely used in many indications such as root-end filling, perforation repair, apexification, and pulp capping.1,2) Traditionally, various materials, such as amalgam, zinc oxide eugenol (ZOE), Intermediate Restorative Material (IRM; Dentsply Caulk, Milford, DE, USA), and super ethoxy benzoic acid (Super EBA) have been used for root-end filling. Types of root-end filling materials have gone through a lot of changes with time. Currently, MTA is mainly used for its excellent sealing ability and biocompatibility.3)
In the past, there had been a lot of studies identifying cell response to MTA.4-6) According to these studies, MTA appears to stimulate cytokine production in human osteoblasts4 and allow good adherence of the cells to the material.5)
MG63 human osteoblast-like cells, originally isolated from a human osteosarcoma, have frequently been used in many experiments. These cells show important osteoblastic traits, including the production of high levels of alkaline phosphatase (ALP) activity and osteocalcin (OC) synthesis. Thus, they were used not only in the study of implant surface,7,8) but also in cellular response to dental materials9) and cytotoxicity test.10,11)
After MG63 cells being attached to root-end filling materials, initial cell response can be evaluated by measuring the amount of ALP and OC,12) biochemical markers showing bone formation, and the amount of TGFβ1,13) growth factor affecting osteoblast through enzyme-linked immunosorbent assay (ELISA).
Recently, new root canal repair filling material, DiaRoot BioAggregate (DB; Innovative BioCaramix Inc, Vancouver, BC, Canada) was developed. According to the manufacturer, this material contains biocompatible pure white powder composed of ceramic nano-particles and deionized water. And powder is composed of hydraulic calcium silicates, calcium phosphate, amorphous silicon oxide and tantalum oxide, contained in a crystalline mass, not separable into individual components.14) Though there was a research on cytotoxic15) and antibacterial16) effect of DB, there are few published studies on its osteogenic effect. So in this study, by comparing DB with highly evaluated existing MTA, potential applicability of this new material as root-end filling material was studied.
MG63 cells, which are osteoblastic cell line, were obtained from the American Type Culture Collection (Rockville, MD, USA). Cultures were maintained in Dulbecco's Modified Eagle's Medium (DMEM; Gibco, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, NY, USA) and 1% penicillin/ streptomycin (Gibco, NY, USA). All experiments were performed using cells between seven and nine passage.
In this study, three kinds of root-end filling materials were used: MTA, DB, IRM. And tissue culture plates with no material were used as control. Each material was mixed according to the manufacturer's instruction. Mixed material was inserted into the wells of 24-well tissue culture plates and condensed to disks that were approximately 1mm thick and had the same diameter as the wells. The materials were allowed to set for 24 hours at 37℃ under 5% CO2 and 100% humidity. And then the cells were seeded on DB, MTA, and IRM in 24-well tissue culture plates in a density of 2 × 105 cells/well. In every experiment, six wells with no disks (plastic controls) and six wells for each of the three types of root-end filling materials were used. For the cells to adhere to each material sufficiently, the cells were grown on different root-end filling materials in DMEM medium for 4 hours and then were changed with new completed DMEM for 12 hours. Attached cells were harvested by trypsinization with 0.25% trypsin and 1mM EDTA. Cells were centrifuged at 1,200 × rpm for 5 min at 4℃ and then stained with trypan blue. Cell number was counted by hemacytometer and the culture supernatants were stored at -80℃ for ELISA.
MG63 cells (2 × 105 cells/well) were seeded into 24-well culture plates and incubated for 12 hours in DMEM supplemented with 10% FBS. The cells were harvested by trypsinization with 0.25% trypsin and 1mM EDTA. After the cells were centrifuged at 1,200 × rpm for 5 min at 4℃, supernatant was removed. 0.5 ml distilled water was added to the cell pellet and this was homogenized with sonication. We measured Protein concentration by BCA assay (Pierce) and ALP with p-nitrophenyl phosphate. Briefly, The cell lysates were incubated with 15 mM p-nitrophenyl phosphate in 0.1 M glycine-NaOH buffer (pH 10.3) at 37℃ for 30 min. Reactions were stopped by the addition of 0.25 N NaOH. The standard concentrations were used p-nitrophenol. The optical density was measured at 405 nm by ELISA reader. The ALP activity was normalized with protein concentration of each aliquot.
The culture supernatants were assayed to determine the cytokine levels according to the manufacturer's instructions. TGFβ1 was measured by ELISA kit obtained from R&D systems (Minneapolis, MN, USA). OC was measured by ELISA kit obtained from Bender medsystems GmbH (Vienna, Austria).
The data are presented from one of the two replicate experiments. Each data point represents the mean ± standard deviation (SD) of six individual cultures. The statistical significance of the differences was analyzed by ANOVA and Bonferroni's t-test. A p-value of less than 0.05 was considered significant.
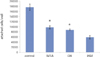
Out of the total number of cells added, 88.13 ± 6.25% had attached to the cell culture plate surface (control) after 12 hours of incubation. On the other specimens the following levels of cell attachment were observed: MTA, 48.75 ± 3.23%; DB, 43.75 ± 1.44%; IRM. 29.38 ± 3.31%. There was no significant difference only between MTA and DB (p > 0.05) (Figure 1, Table 1).
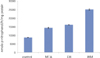
For each item of TGFβ1 and OC, followings are the converted results per 105 cells which were attached to materials. Only for OC, there was no significant difference between MTA and DB (p > 0.05). For remaining others, there was significant difference between every two group (p < 0.05) (Figure 2, 3, Table 2).
Root-end filling is a critical process, which can affect success and failure of apicoectomy, key treatment to surgical endodontic treatment. Important criteria to judge success of surgical endodontic treatment is to confirm bone regeneration at apical lesion through radiograph. Healing after apical surgery includes dentoalveolar healing and alveolar healing.17) Dentoalveolar healing is regeneration of apical attachment apparatus while alveolar healing is osseous repair of medullary and cortical bone. This study focused on finding out relevance between root-end filling materials and alveolar healing. Root-end filling material, which helps bone formation and repair, stimulates alveolar healing, thereby increasing success rates in apical surgery.
Established cell line such as MG63 cells used in this study has advantages in phenotypical consistency, stable cell population, and sufficient biochemical analyses. Reason for using MG63 cells in this study lies in its genetic identity under distinct line and possibility of test reproduction and standardization. In addition, MG63 cells have similar adhesive property and physiology compared with osteoblasts.6) However, this does not always guarantee results from osteosarcoma cell culture since immortalization can affect cellular behavior. In the other experiment primary osteoblasts were more sensitive to cell culture of white MTA than MG63 cells. Therefore, there is an opinion that primary osteoblasts are appropriate model for the study of cellular interactions with endodontic materials.9,18)
MTA, comparable target of this test, has been extensively studied for the past decade at endodontic field. In particular, MTA is widely used for root-end filling after periapical surgery. And there have been a lot of studies on this subject as well. Koh et al. investigated the cytomorphology of osteoblasts (cell-line MG63) in the presence of MTA and IRM and reported good attachment of the cells to MTA.5) Mitchell reported biocompatibility of MTA by using cell growth scoring system in 1999, though he could not make any statistical analysis.6)
This study compared DB, developed as MTA's substitute, with MTA. Cellular reaction was investigated arising from the contact of MG63 cells with several root-end filling materials. As a specific method, ELISA was used to assess cytokine expression. This assay provides good quantification at a biochemical level.
ALP and OC are phenotypic marker of osteoblast and closely related with osteogenesis. In addition, increased TGFβ1 was known to create an osteogenic microenvironment, good to create bone.8) For this reason, concentration TGFβ1 and OC was estimated by ELISA respectively. Original data from ELISA was presented as the amount created by all cells adhered to the materials. Therefore, the resulting value was converted per 105 cells. Like other experiments, tissue culture plastic was used as control while IRM, known to have high cytotoxicity, was used for comparison.
According to the pilot study, substantial number of MG63 cell was adhered to control group in 2 hours after cell-seeding, which was observed by optical microscope. After 4 hours, there was no more adhesion. So medium was exchanged in 4 hours. After another 12 hours of cell culture, attached cell number was counted and ELISA was carried out to observe relatively initial response taken place between root-end filling materials and cells.
As a result of the number of attached cells, 88% of cells seeded at control shows highest adhesion while that of MTA and DB were similar with 49% and 43% respectively. IRM ranked the lowest with 29%. Only between MTA and DB, there was no statistical significance. From this result, MTA and DB's effect on cell adhesion is similar. Moreover, as expected, results showed that cytotoxicity of IRM is relatively higher than that of MTA and DB.
Zhu et al. studied adhesion of human osteoblast (cell line Saos-2) to a number of root-end filling material and showed that osteoblasts were adhered to MTA and composite resin, thereby spreading. However, on IRM osteoblasts appeared rounded with no spreading.19) These results also indicate that MTA is more favorable to osteoblast than IRM.
OC is a major non-collagenous protein of bone matrix and plays a regulatory role in the mineralization of hard tissue. This is synthesized in the bone by the osteoblasts. This is also produced by the cells with mineralizing capacity such as odontoblasts and cementoblasts. Exact physiological function of OC has not been clearly known yet. However, based on a lot of studies, circulating level of OC is known to reflect bone formation rate.20) In the response of cementoblast cell line (OCCM.30) to MTA, amalgam, and IRM, cellular adhesion and growth of cementoblasts on MTA are comparable to this study using osteoblastic cells.21) This results show that MTA allows cementoblastic cell attachment, growth and matrix protein expression involved in mineralization, which is similar in the osteoblastic cells.
TGFβs are members of a superfamily of growth factors that have important roles in the regulation of many aspects of cell growth, proliferation, differentiation and function.22) In vitro effects of TGFβ on osteoblasts have been reported as highly variable and dependent on culture conditions, cell type, and species of origin.
Kassem et al. investigated TGFβ's effects on human osteoblasts with two different stages of differentiation. According to the results, TGFβ1 increased osteoblastic cell proliferation irrespective of differentiation state and increased ALP activity, but decreased OC production. While ALP is produced early during osteoblast differentiation and related to matrix production, OC is produced late and is associated with matrix mineralization. That is to say, ALP is an early marker of osteogenic differentiation and is found in high levels in cells such as osteoblasts.7) And primary effect of TGFβ1 is to increase proliferation of osteoblast in early/intermediate stages of differentiation. TGFβ1 seems to be inhibitory to matrix mineralization as well as late stages of osteoblast differentiation.13) Another study demonstrated that TGFβ1 is an important growth factor for bone formation and physiologically up-regulate differentiation of osteoblasts.23) Thus, increase of TGFβ1 may contribute to elevate ALP activity and production of OC.7)
Since a lot of cytokines, growth factors and hormones are associated with proliferation and differentiation of osteoblast, exact mechanism of contributing factors to bone formation is not completely uncovered, which needs to be further identified.
In the other study, which seeded human periodontal ligament fibroblasts to MTA, production level of cytokines and growth factors in line with time was observed.24) If we get the results based on the various point of time and the production level is estimated at gene level by using PCR, it may be helpful to get more information.
In our results for TGFβ1 and ALP there was statistically significant difference between all groups. In other words, the amount of TGFβ1 and ALP was statistically different even between MTA and DB, but the difference was relatively smaller when compared with the other groups (average of TGFβ1 : control 29, MTA 57, DB 62, IRM 19, average of ALP : control 8747, MTA 14346, DB 16213, IRM 25157).
In conclusion, DB cannot be regarded as showing completely same cellular response compared to MTA. However, there was no difference in the number of attached cells, which show biocompatibility of the material, and in the amount of OC, strong indicator to show the bone formation rate. In that regard, DB can be used for alternative material to MTA as a root-end filling material.
Figures and Tables
References
1. Torabinejad M, Chivian N. Clinical applications of mineral trioxide ag gregate. J Endod. 1999. 25:197–205.
2. Yun YR, Yang IS, Hwang YC, Hwang IN, Choi HR, Yoon SJ, Kim SH, Oh WM. Pulp response of mineral trioxide aggregate, calcium sulfate or calcium hydroxide. J Korean Acad Conserv Dent. 2007. 32:95–101.

3. Schmitt D, Lee J, Bogen G. Multifaceted use of ProRoot MTA root canal repair material. Pediatr Dent. 2001. 23:326–330.
4. Koh ET, Torabinejad M, Pitt Ford TR, Brady K, McDonald F. Mineral trioxide aggregate stimulates a biological response in human osteoblasts. J Biomed Mater Res. 1997. 37:432–439.

5. Koh ET, McDonald F, Pitt Ford TR, Torabinejad M. Cellular response to mineral trioxide aggregate. J Endod. 1998. 24:543–547.

6. Mitchell PJC, Pitt Ford TR, Torabinejad M, McDonald F. Osteoblast biocompatibility of mineral trioxide aggregate. Biomaterials. 1999. 20:167–173.

7. Rausch-fan X, Qu Z, Wieland M, Matejka M, Schedle A. Differentiation and cytokine synthesis of human alveolar osteoblasts compared to osteoblast-like cells(MG63) in response to titanium surfaces. Dent Mater. 2008. 24:102–110.

8. Qu Z, Rausch-fan X, Wieland M, Matejka M, schedle A. The initial attachment and subsequent behavior regulation of osteoblasts by dental implant surface modification. J Biomed Mater Res A. 2007. 82:658–668.

9. Perez AL, Spears R, Gutmann JL, Opperman LA. Osteoblasts and MG-63 osteosarcoma cells behave differently when in contact with ProRoot MTA and white MTA. Int Endod J. 2003. 36:564–570.

10. Schmalz G. Use of cell cultures for toxicity testing of dental materials - advantages and limitations. J Dent. 1994. 22:suppl 2. S6–S11.
11. Kang MK, Bae IH, Koh JT, Hwang YC, Hwang IN, Oh WM. Comparison of biocompatibility of four root perforation repair materials. J Korean Acad Conserv Dent. 2009. 34:192–198.

12. Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev. 1996. 17:333–368.

13. Kassem M, Kveiborg M, Eriksen EF. Production and action of transforming growth factor-β in human osteoblast cultures: dependence on cell differentiation and modulation by calcitriol. Eur J Clin Invest. 2000. 30:429–437.

14. Diadent group international. Material safety data sheet DiaRoot bioAggregate. 2007.
15. De-Deus G, Canabarro A, Alves G, Linhares A, Senne MI, Granjeiro JM. Optimal cytocompatibility of a bioceramic nanoparticulate cement in primary human mesenchymal cells. J Endod. 2009. 35:1387–1390.

16. Zhang H, Pappen FG, Haapasalo M. Dentin enhances the antibacterial effect of mineral trioxide aggregate and bioaggregate. J Endod. 2009. 35:221–224.

17. Apaydin ES, Shabahang S, Torabinejad M. Hard-tissue healing after application of fresh or set MTA as root-end-filling material. J Endod. 2004. 30:21–24.

18. AL-Rabeah E, Perinpanayagam H, MacFarland D. Human alveolar bone cells interact with ProRoot and tooth-colored MTA. J Endod. 2006. 32:872–875.

19. Zhu Q, Haglund R, Safavi KE, Spangberg LSW. Adhesion of human osteoblasts on root-end filling materials. J Endod. 2000. 26:404–406.

20. Bender MedSystems GmbH. BMS2020INST human osteocalcin. 2006.
21. Thomson TS, Berry JE, Somerman MJ, Kirkwood KL. Cementoblasts maintain expression of osteocalcin in the presence of mineral trioxide aggregate. J Endod. 2003. 29:407–412.

22. Cassidy N, Fahey M, Prime SS, Smith AJ. Comparative analysis of transforming growth factor-β isoforms 1-3 in human and rabbit dentine matrices. Arch Oral Biol. 1997. 42:219–223.





 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download