Abstract
Proteoglycan is highly hydrophilic and negatively charged which enable them attract the water. The objective of study was to investigate the effects of Proteoglycan on microtensile bond strength of dentin adhesives and on architecture of dentin collagen matrix of acid etched dentin by removing the chondroitin sulphate attached on Proteoglycan. A flat dentin surface in mid-coronal portion of tooth was prepared. After acid etching, half of the specimens were immersed in 0.1 U/mL chondroitinase ABC (C-ABC) for 48 h at 37℃, while the other half were stored in distilled water. Specimens were bonded with the dentin adhesive using three different bonding techniques (wet, dry and re-wet) followed by microtensile bond strength test. SEM examination was done with debonded specimen, resin-dentin interface and acid-etched dentin surface with/without C-ABC treatment.
For the subgroups using wet-bonding or dry-bonding technique, microtensile bond strength showed no significant difference after C-ABC treatment (p > 0.05). Nevertheless, the subgroup using rewetting technique after air dry in the Single Bond 2 group demonstrated a significant decrease of microtensile bond strength after C-ABC treatment. Collagen architecture is loosely packed and some fibrils are aggregated together and relatively collapsed compared with normal acid-etched wet dentin after C-ABC treatment. Further studies are necessary for the contribution to the collagen architecture of noncollagenous protein under the various clinical situations and several dentin conditioners and are also needed about long-term effect on bond strength of dentin adhesive.
Easy-to-use, efficient and long durable bonding to dentin is a goal that has been endeavored by many researchers, manufacturers and clinicians for a long time. The modern dentin adhesive systems can be classified as the approach to the smear layer: Self-etch approach, Etch-and-Rinse approach.1) The self-etch approach aims to modify the smear layer and incorporate it in the bonding process that involves either two or one-step application, whereas the Etch- and- rinse adhesion strategy involves at least two or three steps with successive application of the acid etchant that result in complete removal of smear layer followed by the primer and application of the adhesive resin. At dentin, phosphoric acid treatment opens the dentin tubules by removing the smear plugs and exposes a microporous network of collagen that is almost free of hydroxyappatite, which the bonding monomers can impregnate. This impregnation process makes a complex hybrid layer formed by residual mineral crystals, collagen fibrils and noncollagenous protein.2) Ideally, a similar volume of adhesive resin should replace the volume of mineral removed from dentin during the demineralization process. However, a decreasing gradient of resin monomer diffusion within the acid etched dentin results in incompletely infiltrated zones along the bottom of the hybrid layer in the demineralized zone of dentin by discrepancy between the depth of acid etching and resin infiltration.3) This incompletely infiltrated zone produce the nanoleakage that may be the pathway that cause degradation of the resin-dentin interface by hydrolysis of collagen fibrils and polymerized resin.4-6)
In order to enhance the optimal formation of a hybrid layer, demineralized dentin has to be kept moist and collagen network maintained in a fully expanded state after acid etching or if dried, need to be re-wetting.7) The presence of water play a crucial role in preventing the demineralized surface collagen from collapsing following dehydration.8) When air-dried demineralized dentin is re-wet with water, the collagen matrix re-expands and completely recovers the original dimensions to the levels of the original hydrated state7) because spaces between fibrils is refilled with water and hydrogen bond between collagen fiber that restrain the adhesive resin infiltration is broken by water.10,11)
Unlike enamel, of which the organic matrix makes up only about 0.4-0.6%, dentin contains approximately 20% organic component in weight.12) Type I collagen constitutes about 90% of the dentin organic matrix and the remaining 10% of dentin organic matrix is composed by a number of non-collagenous proteins (NCPs). Proteoglycans (PGs) and phosphoproteins comprise of the main components of the dentin noncollagenous protein.13)
PGs are composed of a core protein and one or more glycosaminoglycans (GAGs) chains covalently attached to it. The side chains of GAGs attached to the core proteins demonstrate the functional characteristics of PGs14) and two of the most prominent PGs in dentin are decorin and biglycan, which are relatively abundant and usually carry chondroin sulphate GAG chain.15)
PGs are highly hydrophilic and negatively charged which enable them attract the water due to carboxyl and sulfate groups present in GAG chains and have electrostatic interaction with collagen,15-17) also are susceptible to degradation both by matrix metalloproteinases (MMPs),18) bacterial enzymes,20) and show the decreased antigenicity when 35% phosphoric acid was applied on dentin surface for more than 30s.21) Those characteristics of PGs may play an important role in the maintenance of dentin collagen architecture and contribute to the bond strength of dentin adhesive and durability of bonding interface.
The aim of this study was to investigate the effects of Proteoglycan on microtensile bond strength of dentin adhesives and on architecture of dentin collagen matrix of acid etched dentin by removing the chondroitin sulphate that is most abundant glycosaminoglycan in dentin.
Seventy-two, caries-free, recently extracted human third molars were stored in a 0.5% chloramines T aqueous solution at 4℃ and used within 1 month following extraction. A flat dentin surface in mid-coronal portion of tooth was prepared perpendicular to the longitudinal axis of each tooth using a slow-speed Isomet diamond saw (Buehler Ltd., Lake Bluff, IL, USA) under copious water-cooling. Each dentin surfaces were then polished with a 600-grit silicon carbide paper under running water to creat a bonding surface with a standardized smear layer in mid-coronal dentine that was devoid of enamel. The teeth were randomly divided into two groups (N=36) according to the dentin adhesives used: Single Bond 2 (3M ESPE, St. Paul, MN, USA), a water-containing, ethanol-based etch-and-rinse adhesive, and One Step plus (Bisco Inc., Schaumburg, IL, USA), a water-free, acetone-based etch-and rinse adhesive. Composition, batch number and manufacturers of the tested adhesives are listed in Table 1. Each dentin surface to be bonded was etched with a 37% phosphoric acid gel (3M ESPE, St. Paul, MN) for 15 s to create a 5-8 µm thick zone of completely demineralized dentin. The etched dentin surface was then rinsed with deionized water for 10 s and kept visibly moist to prevent collapse of the collagen matrix. Prior to the bonding procedures, the specimens of each group were randomly divided into two subgroups according to solution that was used for the pretreatment of acid-etched dentin surfaces: Half of the specimens in each group were immersed in 0.1 U/mL chondroitinase ABC (C-ABC) (Sigma chemical, St. Lois, MO.) in 0.1 M Tris/Acetate (pH 7.5) for 48 h (renewed after 24 h) at 37℃, while the other half were stored in distilled water under the same conditions. After incubation for 48 h, all specimens were thoroughly rinsed with distilled water for 5 min and bonded with the respective adhesive using three different bonding techniques (N=3 for each subgroup): I) moist dentin was blot dried; II) moist dentin was air dried for 15 s; and III) moist dentin was air dried for 15 s, re-wetted for 30 s and subsequently blot dried. The dentin adhesives were used in accordance with manufacturer's instructions. This was followed by incremental placement of a composite resin (Z-250, 3M ESPE, St. Paul, MN, USA) to a height of 5 mm. Light curing was performed with a Spectrum 800 halogen light-curing unit (Dentsply DeTrey, Konstanz, Germany) with power output of 600 mW/cm2.
After water-storage at 37℃ for 24 h, each tooth was sectioned occluso-gingivally into 1 mm thick serial slabs (N=6 for each teeth) by means of the slow speed diamond saw with water-cooling. The central slab was used for SEM examination, while the others were further sectioned into 1 × 1 mm beams, using the "non-trimming"technique of the microtensile testing. The two longest beams from each slab were selected for tensile testing, yielding 30 beams per subgroup. Specimens that failed prematurely during sectioning were recorded in each subgroup, and the beam dimension of each intact specimen was measured with digital caliper to the nearest 0.01 mm to provide the bonding surface area for calculating microtensile bond strength. Each beam was fixed by their ends to the microtensile bond testing device with cyanoacrylate glue (Zapit, Dental Ventures of America, Corona, CA, USA) and tested in tension using material tester (EZ tester, Shimadzu Co., Kyoto, Japan) at a crosshead speed of 1.0 mm/min. Microtensile bond strength was determined in MPa by calculating the quotient of maximum load and the its corresponding bonding area.
Statistical analysis was performed separately for each dentin adhesive since the intention of the study was not compare bond strengths of the two adhesives. Bond strength data was normally distributed (Kolmogorov-Smirnov test) and exhibited equal variances (Levene test). Thus, the data were analyzed using a two-way analysis of variance (ANOVA) for evaluating the effects of enzyme digestion and dentin condition, followed by Duncan test for post-hoc comparisons. Statistical analysis was performed using SPSS 11.0 for window (SPSS Inc., Chicago, IL, USA) with the level of significance set at 5%.
Two representative debonded beams of the mean bond strength of each subgroup were prepared for the observation of the fracture pattern.
Moreover, the central slabs of each specimen were serially polished with 600, 1200, 1500 grit silicon carbide paper under running water, followed by a 5-sec 37% phosphoric acid gel (3M ESPE, St. Paul, MN, USA) treatment and a 10-min 5.25% sodium hypochlorite treatment. The selected beams and slabs were dried for 24 h within the drierite desiccator (Hammond Drierite Company LTD., Xenia, Ohio, USA).
Another eight caries-free, human third molars were used to evaluate change of the collagen architecture after removal of the chondroitin sulfate in proteoglycan. Specimen preparation was carried out according to the method proposed by Perdigao et al.22) in order to avoid artifacts formation due to dehydration shrinkage of specimens and their observation under a high to ultra-high vacuum.21) Fourteen 0.9 mm-thick dentin disks parallel to the occlusal surface were obtained from mid-coronal dentin that was devoid of enamel by low speed diamond saw. A transverse groove was made in the bottom surface of each dentin disk to facilitate splitting after specimen preparation, while its upper surface was etched with a 37% phosphoric acid gel for 15 s, rinsed with water for 10 s and blot dried. All the specimens were randomly distributed into two groups (N=4): one group was then treated with chondroitinase-ABC (C-ABC) and the other group was stored in distilled water as the same way described in the microtensile test section. After C-ABC/distilled water storage, specimens of each group were subdivided into two subgroups (N=2) according to dentin moisture, namely wet, and rewet. The specimens were immediately immersed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer at pH 7.4 for 4 hours at 4℃. After fixation, the disks were rinsed with 0.1 M sodium cacodylate buffer at pH 7.4 for 1 h, with three changes, followed by distilled water for 5 min. After dehydration in ascending ethanol series (50-100%), the specimens were HMDS-dried. Specimens for examination of sagittal surface of acid-etched dentin were split with forceps before mounting the stubs.
After dried, all of SEM specimens were mounted in aluminum stubs with carbon tape. They were sputter-coated with platinum by means of sputter-coating Unit (E-1045, Polaron, Germany) and examined with a FE-SEM (SUPRA 55, Zeiss, Germany) at an acceleration voltage of 5-10 kV.
Premature failure (PF) was observed in all groups of air-dried dentin and Single Bond 2 groups pretreated with C-ABC. The data for microtensile bond strength and percentage of premature failure of each experimental group are summarized in Table 2. For One Step plus, the bond strengths in descending order were wed-bonding subgroups, subgroups using rewetting technique, and dry-bonding subgroups. The same was true when the Single Bond 2 group was taken into consideration. Two-way ANOVA indicated a significant difference in the bond strengths of each dentin adhesives among different moisture conditions of dentin (p < 0.05), whilst there was no significant difference between dentin with and without C-ABC treatment (p > 0.05), and showed that the interaction between dentin condition and C-ABC Treatment was significant for Single Bond 2 only (p = 0.002), not for One Step plus (p > 0.05).
For the subgroups using wet-bonding or dry-bonding technique, microtensile bond strength showed no significant difference after C-ABC treatment (p > 0.05). Nevertheless, the subgroup using rewetting technique after air dry in the Single Bond 2 group demonstrated a significant decrease of microtensile bond strength after C-ABC treatment compared with its counterpart without C-ABC treatment (p < 0.05).
Premature failure (PF) was observed in groups of dry-bonding technique in both tested dentin adhesives and C-ABC pretreated groups in Single Bond 2 (Figure 1).
Representative FE-SEM images of fractured specimens were selected and shown in Figure 2. Images of resin-dentin interface were shown in Figure 3 and image of collagen architecture were shown in Figure 4, 5. For Single Bond 2, failure occurred within the adhesive resin to the bottom of the hybrid layer and dentinal tubules were occluded with the resin tag (Figure 2a). For One Step Plus, failure was occurred within the densely formed hybrid layer (Figure 2c). After C-ABC treatment, failures were occurred at the bottom of hybrid layer with resin tag exclusion from dentinal tubules at both dentin adhesives (Figure 2b, 2d).
Regardless of dentin adhesive, thickness of hybrid layer was increased in C-ABC treated group compared with not treated group when adhesives were used to wet dentin (Figure 3).
Regarding FE-SEM observation with high magnification of the collagen architecture, the collagen of normal acid-etched wet dentin is uniformly arranged and compact interfibrillar space and fully expanded state (Figure 4a, 4b, Figure 5a). After C-ABC pretreatment, collagen architecture is loosely packed and some fibrils are aggregated together and relatively collapsed compared with normal acid-etched wet dentin (Figure 4c, 4d, Figure 5b, 5c).
In general, PGs are considered to play structural and metabolic functional roles in both soft and mineralized tissues of body, and are present in a wide variety of non-vertebrate and vertebrate species.24) They consist of glycosaminoglycan (GAG) chains, comprising repeating disaccharides units, covalently linked to a protein.15) The GAG chains are mainly classified into five types: hyaluronan, heparin/heparan sulphate, chondroitin sulfate, dermatan sulphate and keratan sulphate, previously illustrated in predentin, dentin,25,26) cementum, periodontal ligament27) and cementum-dentin junction27) of teeth. Biochemical and immunohistochemical studies on predentin and dentin PGs indicated that PGs predominantly belong to the small leucine-rich PG (SLRP) family14) and the distinctive feature of the SLRP is the presence of between 7 and 24 leucinerich tandem repeats in the core protein. Most prominent member of dentin PGs are decorin and biglycan, which are identified in various tissues such as skin, tendon, gingival and bone. Decorin usually carries one GAG chain at the fourth amino acid at the N-terminal where chondroitin sulfate (CS) predominates within mineralized tissue and dermatan sulphate (DS) in soft tissues. Biglycan usually two GAG chains with CS being predominant in mineralized tissue like decorin. Because immunohistochemical labeling of decorin was more intensive than biglycan in dentin and significantly higher over the odontoblast process compared to the intertubular dentin14) and decreasing gradient toward superficial dentin.24) The removing effect of chondroitinase ABC (C-ABC) that acts on chondroitin 4-sulfate, chondroitin 6-sulfate, and dermatan sulfate, and acts slowly on hyaluronate (Product information, Sigma chemical, St. Lois, MO.), to dentin might be more prominent in the deep dentin.
Dentin is composed of collagen based organic matrix with hydroxyapatite reinforcement that removed by acid etching in total-etch technique. Exposed collagen fibrils are linked by protein core of small leucine-rich proteoglycans (PGs) at the gap zone and they are interacting with each other via their glycosamonoglycans (GAGs) side chains, like a step of the ladder.29,30) The unbranched polysaccharide chains of the GAG, which contain many carboxylate and sulfate group, make the GAG chains polyanionic with a high affinity for water molecules. These GAG-water hydrogel and orthogonal alignment of GAG with collagen fibrils make PGs behave like a stress transfer and distribute the functional tensile and compressive load.31,32) But in current study, digestion of acid-etched dentin matrix with C-ABC for 48 h did not make a significant difference in microtensile bond strength of dentin adhesives regardless of dentin condition, except Single bond 2 with re-wet technique. Thus, it can be speculated that removal of chondroitin sulphate from a hydration state of acid-etched dentin collagen matrix may not have a significant influence on bond strength of dentin adhesives tested.
According to our knowledge, there have been few studies investigating the effect of C-ABC on dentin and it is still a matter of controversy.33,34)
Mazzoni et al. showed the increased values of microtensile bond strength, reduced nanoleakage expression after C-ABC treatment of the dentin surface. Acetone based Prime and Bond NT increased almost doubled (92%) while ethanol-water based Adper Scotchbond Multi-Purpose increased about 28%. In their study, field emission in-lens scanning electron microscopy images of C-ABC treated dentin showed the wider empty spaces between the collagen fibrils, which were consistent with our findings (Figure 4d) and authors thought that those empty spaces act as the diffusion channels for more optimal monomer infiltration making the homogenous hybrid layer.
Elliott and co-workers studied the absence of PGs in transgenic mouse-tail tendons, and their result demonstrated that the collagen network was loosely packed with irregular contours and fibrils fused together.35)
On the contrary, C-ABC treated dentin revealed a significant loss of collagen fibril architecture and bond strengths for the rewetted group in the study of Pereira et al.34) When dentin was kept moist, there is no significant difference of bond strength after CABC treatment like our study while bond strength to dentin digested with C-ABC had significantly lower bond strength compared to its control when the dentin was dried and rewetted. In the SEM analysis of pereira's experiment, loss of interfibrillar space was observed for the C-ABC rewetted group and this might be indicated the role of proteoglycan during the rewetting the dried dentin collagen. This decrease of bond strength in rewet dentin condition was coincided with our Single Bond 2 group. When the demineralized dentin was air-dried and rewetted by water, bond strength was returned to the range obtained on moist dentin even if there is a difference of rewetting time according to the type of solvent used in dentin adhesives.36) It could be possible because the water that is able to break the interpeptide H-bonds between the collapsed dentin collagen make the interfibrillar space that required as diffusion channels for resin infiltration re-expanded.37) During the re-expanding process with water, if hydrophilic proteoglycan between collagen networks was depleted by C-ABC or endogenous matrix metalloproteinase (MMP)18,19) and bacterial enzyme20), this could restrict the breaking ability of interpeptide H-bond of water to the collapsed collagen network, thus, decreased the bond strength in rewetting dentin condition.
When the dentin was adhered with One-step plus, bond strength of C-ABC treated dentin after rewetting with water showed non-significant change compared with non C-ABC pretreated group. These differences of bond strength after rewetting the C-ABC treated dentin between dentin adhesives may be related with the solvent used in each dentin adhesives. Water-chasing ability of acetone38) may explain why acetone-based One step plus showed the more stable results regardless of the dentin condition except air-dried dentin surface than ethanol-based Single Bond 2.
Dehydration of dentin causes the collagen matrix collapse that results in the interfibrillar space that are needed as diffusion channels for dentin adhesive disappear. The collapsed dentin matrix does not expand when dentin adhesives are applied and eventually show the reduced bond strength and clinical symptoms of patients.10) When the dentin surface was air-dried after the acid-etching procedure, all of dentin adhesives tested in our study also showed the significantly decreased bond strength compared with the bond strength obtained in the wet surface and re-wetted surface regardless of treatment of C-ABC.
A comparison of the FE-SEM of the dentin side of the failed bonds of dentin adhesives showed that adhesive failure including the resin tag exclusion mostly occurred in C-ABC treated wet dentin while mixed failure occurred in normal wet dentin even though there is no significant difference of bond strength between the normal wet dentin and C-ABC treated wet dentin. This may indicate the incomplete resin impregnation to the base of hybrid layer and into the dentinal tubule. Such a characteristic was more prominent for Sing Bond 2 that showed the premature failure for the wet group and re-wet group after C-ABC treatment. According to the atomic force microscope study of S.P. Ho et al.,17) the use of C-ABC removed GAGs from CDJ resulting in breakdown of the collagen-PG network. This caused a collapse of the collagen network observed under dry condition and, under wet condition, the loss of structure resulted in an increase in height of the collagen architecture. This increasing height of the collagen network was shown indirectly through the examination of the representative specimens perpendicularly sectioned the dentin-resin interface in our experiment (Figure 3b, 3d). But vertical architecture of collagen after C-ABC pre-treatment is slightly reduced in the FE-SEM observation of fracture surface (Figure 5b). This difference might be caused by collapse of the chondroitin sulfate depleted collagen network which does not occupied by adhesive resin and does not connected by PGs between collagen fibers during the FE-SEM specimen processing.
Higher rate of premature failure of Single Bond 2 compared with One Step plus can be contributed to polyalkenoic acid copolymer as well as different solvents used in each system. Because this copolymer was found to provide better moisture stability to ScotchBond MultiPurpose (SBMP) (3M ESPE, St. Paul, MN, USA),39) it was added to the SBMP primer and Single Bond 2. Due to the significant difference in molecular weight between HEMA (MW = 130, Sigma, St. Louis, MO) and the copolymer (MW = 14,000 to 20,000), while HEMA may diffuse more readily into the collagen network than the copolymer, polyalkenoic copolymer may have been kept at the surface of hybrid layer,40) resulting in amorphous electron-dense phase and consequently, may interfere with the diffusion of adhesive solution to optimize the hybridization, especially in C-ABC treated dentin which collagen network was partially coagulated.
In summary, within the limitation of this study, it is concluded that the selective removal of chondroitin sulphate in acid-etched dentin does not affect the bond strength of dentin adhesives used to wet dentin surface. But, once acid-etched dentin surface was dried, resulting in dentin collagen matrix collapse, and re-wetted with water in clinical situation, impairment of the GAGs may disturb the full reexpansion of collagen matrix and, as a result, the bond strength to dentin may be decreased according to the dentin adhesives.
The study about the role of noncollagenous protein in the adhesive dentistry is very few and just commenced. Further studies are necessary for the contribution to the collagen architecture of noncollagenous protein under the various clinical situations and several dentin conditioners and are also needed about long-term effect on bond strength of dentin adhesive.
Figures and Tables
 | Figure 1Results of microtensile bond strength value (MPa) of each dentin adhesives.
W: wet dentin not treated with enzyme (C-ABC) was blot dried. D: dentin not treated with enzyme (C-ABC) was air dried for 15 s. RW: dentin not treated with enzyme (C-ABC) was air dried for 15 s and then re-wetted for 30 s with wet gauze and blot dried. E-W: wet dentin treated with enzyme (C-ABC) was blot dried. E-D: dentin treated with enzyme (C-ABC) was air dried for 15 s. E-RW, dentin treated with enzyme (C-ABC) was air dried for 15 s and then re-wetted for 30 s with wet gauze and blot dried.
|
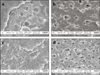 | Figure 2Field Emission-Scanning Electron Microscope (FE-SEM) observations of the fractured surfaces in dentin side after microtensile bond strength testing (Mag: ×10,000).
a. Dentin side produced by Single bond 2 without C-ABC treatment. Failure was occurred within the adhesive resin to the bottom of the hybrid layer. Dentinal tubules were occluded with the resin tag.
b. Dentin side produced by Single bond 2 with C-ABC treatment. Failure was occurred at bottom of hybrid layer with resin tag exclusion. c. Dentin side produced by One Step Plus without C-ABC treatment. Failure was occurred from the top of the hybrid layer to the bottom. The hybrid layer is densely formed.
d. Dentin side produced by One Step Plus with C-ABC treatment. Failure was occurred at bottom of hybrid layer with resin tag exclusion.
|
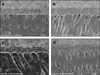 | Figure 3Field Emission-Scanning Electron Microscope (FE-SEM) observations of the resin-dentin interfaces (Mag: ×2,000).
a. Photomicrograph of resin-dentin interface of Single Bond 2 without C-ABC treatment. Hybrid layer of about 6µm thickness is observed.
b. Photomicrograph of resin-dentin interface of Single Bond 2 with CABC treatment. A thicker hybrid layer about 10µm thickness can be seen.
c. Photomicrograph of resin-dentin interface of One Step Plus without C-ABC treatment. Hybrid layer of about 4~6µm thickness is observed.
d. Photomicrograph of resin-dentin interface of One Step Plus with CABC treatment. A thicker hybrid layer about 10µm thickness can be seen like Single bond 2.
|
 | Figure 4Field Emission-Scanning Electron Microscope (FE-SEM) observations of acid-etched dentin surfaces.
a. Collagen fibril architecture of acid-etched wet dentin without C-ABC pre-treatment.
b. A high-magnification view. Well-defined collagen fibrils are arranged with compact interfibrillar space.
c. Collagen fibril architecture of acid-etched wet dentin with C-ABC pre-treatment.
d. A high-magnification view. Collagen architecture is loosely packed with irregular diameter and some fibrils are fused together.
|
 | Figure 5Field Emission-Scanning Electron Microscope (FE-SEM) observations of the vertically fractured dentin surface.
a. Collagen fibrils vertical architecture of acid-etched wet dentin without C-ABC treatment. Collagen network is fully expanded state.
b. Collagen fibrils vertical architecture of acid-etched wet dentin with C-ABC treatment. The collagen network is slightly collapsed compared with wet dentin without C-ABC treatment.
c. Collagen fibrils vertical architecture of acid-etched re-wetted dentin with C-ABC pre-treatment. Collagen network is relatively collapsed compared with normal acid-etched wet dentin.
|
References
1. Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, Van Landuyt K, Lambrechts P, Vanherle G. Buonocore memorial lecture. Adhesion to enamel and dentin: current status and future challenges. Oper Dent. 2003. 28(3):215–235.
2. Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982. 16(3):265–273.

3. Wang Y, Spencer P. Quantifying adhesive penetration in adhesive/dentin interface using confocal Raman microspectroscopy. J Biomed Mater Res. 2002. 59(1):46–55.

4. Sano H, Takatsu T, Ciucchi B, Horner JA, Matthews WG, Pashley DH. Nanoleakage: leakage within the hybrid layer. Oper Dent. 1995. 20(1):18–25.
5. Jang JH, Lee KW, Kim HY, Lee IB, Cho BH, Son HH. Quatitative comparision of permeability in the adhesive interface of four adhesive systems. J Kor Acad Con Dent. 2009. 34(1):55–60.
6. Son SJ, Jang JH, Kang SH, Yoo HM, Cho BH, Son HY. The nanoleakage patterns of experimental hydrophobic adhesives after load cycling. J Kor Acad Cons Dent. 2008. 33(1):9–19.

7. Perdigao J, Van Meerbeek B, Lopes MM, Ambrose WW. The effect of a re-wetting agent on dentin bonding. Dent Mater. 1999. 15(4):282–295.

8. Gwinnett AJ. Dentin bond strength after air drying and rewetting. Am J Dent. 1994. 7(3):144–148.
9. Carvalho RM, Yoshiyama M, Pashley EL, Pashley DH. In vitro study on the dimensional changes of human dentine after demineralization. Arch Oral Biol. 1996. 41(4):369–377.

10. Pashley DH, Agee KA, Nakajima M, Tay FR, Carvalho RM, Terada RS, Harmon FJ, Lee WK, Rueggeberg FA. Solvent-induced dimensional changes in EDTA-demineralized dentin matrix. J Biomed Mater Res. 2001. 56(2):273–281.

11. Agee KA, Becker TD, Joyce AP, Rueggeberg FA, Borke JL, Waller JL, Tay FR, Pashley DH. Net expansion of dried demineralized dentin matrix produced by monomer/alcohol saturation and solvent evaporation. J Biomed Mater Res A. 2006. 79(2):349–358.

12. Linde A. Dentin matrix proteins: composition and possible functions in calcification. Anat Rec. 1989. 224(2):154–166.

13. Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res. 2006. 85(1):22–32.

14. Orsini G, Ruggeri A Jr, Mazzoni A, Papa V, Mazzotti G, Di Lenarda R, Breschi L. Immunohistochemical identification of decorin and biglycan in human dentin: a correlative field emission scanning electron microscopy/transmission electron microscopy study. Calcif Tissue Int. 2007. 81(1):39–45.

15. Goldberg M, Takagi M. Dentine proteoglycans: composition, ultrastructure and functions. Histochem J. 1993. 25(11):781–806.

16. Scott JE. Proteoglycan:collagen interactions and subfibrillar structure in collagen fibrils. Implications in the development and ageing of connective tissues. J Anat. 1990. 169(1):23–35.
17. Ho SP, Sulyanto RM, Marshall SJ, Marshall GW. The cementum-dentin junction also contains glycosaminoglycans and collagen fibrils. J Struct Biol. 2005. 151(1):69–78.

18. Hall R, Septier D, Embery G, Goldberg M. Stromelysin-1 (MMP-3) in forming enamel and predentine in rat incisor-coordinated distribution with proteoglycans suggests a functional role. Histochem J. 1999. 31(12):761–770.
19. Oh EW, Choi KK, Kim JR, Park SJ. Effect of chlohexidine on microtensile bond strength of dentin bonding systems. J Kor Acad Cons Dent. 2008. 33(2):148–161.

20. Smith AJ, Wade W, Addy M, Embery G. The relationship between microbial factors and gingival crevicular fluid glycosaminoglycans in human adult periodontitis. Arch Oral Biol. 1997. 42(1):89–92.

21. Breschi L, Lopes M, Gobbi P, Mazzotti G, Falconi M, Perdigao J. Dentin proteoglycans: an immunocytochemical FEISEM study. J Biomed Mater Res. 2002. 61(1):40–46.

22. Van Meerbeek B, Vargas M, Inoue S, Yoshida Y, Perdigao J, Lambrechts P, Vanherle G. Microscopy investigations. Techniques, results, limitations. Am J Dent. 2000. 13(Spec No):3D–18D.
23. Perdigao J, Lambrechts P, Van Meerbeek B, Vanherle G, Lopes AL. Field emission SEM comparison of four postfixation drying techniques for human dentin. J Biomed Mater Res. 1995. 29(9):1111–1120.

24. Embery G, Hall R, Waddington R, Septier D, Goldberg M. Proteoglycans in dentinogenesis. Crit Rev Oral Biol Med. 2001. 12(4):331–349.

25. Goldberg M, Septier DS. Differential staining of glycosaminoglycans in the predentine and dentine of rat incisor using cuprolinic blue at various magnesium chloride concentrations. Histochem J. 1992. 24(9):648–654.

26. Hall RC, Embery G, Lloyd D. Immunochemical localization of the small leucine-rich proteoglycan lumican in human predentine and dentine. Arch Oral Biol. 1997. 42(10-11):783–786.

27. Kagayama M, Sasano Y, Mizoguchi I, Kamo N, Takahashi I, Mitani H. Localization of glycosaminoglycans in periodontal ligament during physiological and experimental tooth movement. J Periodontal Res. 1996. 31(4):229–234.

28. Yamamoto T, Domon T, Takahashi S, Islam MN, Suzuki R. The fibrous structure of the cemento-dentinal junction in human molars shown by scanning electron microscopy combined with NaOH-maceration. J Periodontal Res. 2000. 35(2):59–64.

30. Raspanti M, Congiu T, Alessandrini A, Gobbi P, Ruggeri A. Different patterns of collagen-proteoglycan interaction: a scanning electron microscopy and atomic force microscopy study. Eur J Histochem. 2000. 44(4):335–343.
31. Bartold PM, Miki Y, McAllister B, Narayanan AS, Page RC. Glycosaminoglycans of human cementum. J Periodontal Res. 1988. 23(1):13–17.

32. Redaelli A, Vesentini S, Soncini M, Vena P, Mantero S, Montevecchi FM. Possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons--a computational study from molecular to microstructural level. J Biomech. 2003. 36(10):1555–1569.

33. Mazzoni A, Pashley DH, Ruggeri A Jr, Vita F, Falconi M, Di Lenarda R, Breschi L. Adhesion to chondroitinase ABC treated dentin. J Biomed Mater Res B Appl Biomater. 2008. 86(1):228–236.

34. Pereira PN, Bedran-de-Castro AK, Duarte WR, Yamauchi M. Removal of noncollagenous components affects dentin bonding. J Biomed Mater Res B Appl Biomater. 2007. 80(1):86–91.

35. Elliott DM, Robinson PS, Gimbel JA, Sarver JJ, Abboud JA, Iozzo RV, Soslowsky LJ. Effect of altered matrix proteins on quasilinear viscoelastic properties in transgenic mouse tail tendons. Ann Biomed Eng. 2003. 31(5):599–605.

36. Perdigao J, Frankenberger R. Effect of solvent and rewetting time on dentin adhesion. Quintessence Int. 2001. 32(5):385–390.
37. Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, Donnelly A, Garcia-Godoy F. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent. 2007. 20(1):7–20.
38. Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechts P, Van Meerbeek B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007. 28(26):3757–3785.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download