Abstract
The purpose of this study is to investigate the response of human pulp cell on Portland cement mixed with β-glycerophosphate. To investigate the effect of β-glycerophosphate and/or dexamethasone on human pulp cell, ALP activity on various concentration of β-glycerophosphate and dexamethasone was measured and mineral nodule of human pulp cell was stained with Alizarin red S. MTS assay and ALP activity of human pulp cell on Portland cement mixed with various concentration of β-glycerophosphate (10 mM, 100mM, 1M) was measured and the specimens were examined under SEM.
Addition of β-glycerophosphate or dexamethasone alone had no effect however, the addition of 5 mM β-glycerophosphate and 100 nM dexamethasone had the largest increasement in ALP activity. There was no toxicity in all samples and the data showed that Portland cement mixed with 10 mM β-glycerophosphate had more increase in ALP activity compared with control.
In conclusion, Portland cement mixed with β-glycerophosphate has no toxicity and promotes differentiation and mineralization of pulp cell compared with additive-free Portland cement. This implicated that application of Portland cement mixed with β-glycerophosphate might form more reparative dentin and in turn it would bring direct pulp capping to success.
Figures and Tables
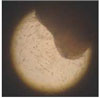
 | Figure 11SEM evaluation of pulp cell on Portland cement mixed with β-glycerophosphate at 1 day. A: Portland cement with no β-glycerophosphate. B, C, D: Portland cement with β-glycerophosphate 10 mM, 100 mM, 1 M. |
References
1. Gronthos S, Mankani M, Brahim J, Gehron PR, Shi S. Postnatal human dental pulp stem cells(DPSCs) in vitro and in vivo. Proc Natl Acad Sci. 2000. 97:13625–13630.
2. About I, Bottero MJ, de Denato P, Camps J, Franquin JC, Mitsiadis TA. Human dentin production in vitro. Exp Cell Res. 2000. 258:33–41.

3. Laino G, d'Aquino R, Graziano A, Lanza V, Carinci F, Naro F, Pirozzi G, Papaccio G. A New population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res. 2005. 20:1394–1402.

4. Couble ML, Farges JC, Bleicher F, Perrat-Mabillon B, Boudeulle M, Magloire H. Odontoblast differentiation of human dental pulp cells in explant cultures. Calcif Tissue Int. 2000. 66:129–138.

5. Huang GT, Shagramanova K, Chan SW. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod. 2006. 32:1066–1073.

6. Yun YR, Yang IS, Hwang IN, Choi HR, Yoon SJ, Kim SH, Oh WM. Pulp Response of Mineral Trioxide Aggregate, Calcium Sulfate or Calcium Hydroxide. J Korean Acad Conserv Dent. 2007. 32:95–101.

7. Moghaddame-Jafari S, Mantellini MG, Botero TM, McDonald NJ, Nör JE. Effect of ProRoot MTA on Pulp Cell Apoptosis and Proliferation In Vitro. J Endod. 2005. 31:387–391.

9. Fridland M, Rosado R. Mineral Trioxide Aggregate (MTA) Solubility and Porosity with Different Water-to-Powder Ratios. J Endod. 2003. 29:814–817.

10. Estrela C, Bammann LL, Estrela CR, Silva RS, Pécora JD. Antimicrobial and chemical study of MTA,Portland cement, calcium hydroxide paste, Sealapex and Dycal. Braz Dent J. 2000. 11:3–9.
11. Glasser FP. Fundamental aspects of cement solidification and stabilisation. J Hazard Mater. 1997. 52:151–170.

12. Min KS, Kim HI, Park HJ, Pi SH, Hong CU, Kim EC. Human Pulp Cells Response to Portland Cement In Vitro. J Endod. 2007. 33:163–166.

13. Cotton WR. Finn SD, editor. Pulp response to cavity preparation as studies by the method of thymidine 3H autoradiography. Biology of the dental pulp organ. 1968. Tuscaloosa, AL: Univ Alabama Press;69.
14. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003. 100:5807–5812.

15. Pincus P. Some physiological data on the human dental pulp. Br Dent J. 1950. 89:143–148.
16. Tjäderhane L, Salo T, Larjava H, Larmas M, Overall CM. Novel Organ Culture Method to study the Function of Human Odontoblasts in vitro: Gelatinase Expression by Odontoblast is Differentially Regulated by TGF β1. J Dent Res. 1998. 77:1486–1496.

17. Ishikawa H, Kitoh H, Sugiura F, Ishiguro N. The effect of recombinant human bone morphogenetic protein-2 on the osteogenic potential of rat mesenchymal stem cells after several passages. Acta Orthopaedica. 2007. 78:285–292.

18. Arany S, Nakata A, Kameda T, Koyota S, Ueno Y, Sugiyama T. Phenotype properties of a novel spontaneously immortalized odontoblast-lineage cell line. Biochem Biophys Res Commun. 2006. 342:718–724.

19. Alliot-Licht B, Bluteau G, Magne D, Lopez-Cazaux S, Lieubeau B, Daculsi G, Guicheux J. Dexamethasone stimulates differentiation of odontoblast-like cells in human dental pulp cultures. Cell Tissue Res. 2005. 321:391–400.

20. Fratzl-Zelman N, Fratzl P, Horandner H, Grabner B, Varga F, Ellinger A, Klaushofer K. Matrix Mineralization in MC3T3-E1 Cell Cultures Initiated by β-Glycerophosphate Pulse. Bone. 1998. 23:511–520.

21. Tang AT, Li J, Ekstrand J, Liu Y. Cytotoxicity tests of in situ polymerized resins: Methodological comparisons and introduction of a tissue culture insert as a testing device. J Biomed Mater Res. 1999. 45:214–222.

22. Bidar M, Moradi S, Jafarzadeh H, Bidad S. Comparative SEM study of the marginal adaptation of white and grey MTA and Portland cement. Aust Endod J. 2007. 33:2–6.

23. De-Deus G, Petruccelli V, Gurgel-Filho E, Coutinho-Filho T. MTA versus Portland cement as repair material for furcal perforations: a laboratory study using a polymicrobial leakage model. Int Endod J. 2006. 39:293–298.

24. Ribeiro DA, Duarte MA, Matsumoto MA, Marques ME, Salvadori DM. Biocompatibility in vitro tests of mineral trioxide aggregate and regular and white Portland cements. J Endod. 2005. 31:605–607.

25. Chang SW, Yoo HM, Park DS, Oh TS, Bae KS. Ingredients and cytotoxicity of MTA and 3 kinds of Portland cement. J Korean Acad Conserv Dent. 2008. 33:369–376.

26. Danesh G, Dammaschke T, Gerth HU, Zandbiglari T, Schäfer E. A comparative study of selected properties of ProRoot mineral trioxide aggregate and two Portland cements. Int Endod J. 2006. 39:213–219.





 PDF
PDF ePub
ePub Citation
Citation Print
Print










 XML Download
XML Download