I. INTRODUCTION
Vital bleaching is one of the most conservative, simple, economical, and painless measure to lighten teeth
1). In-office bleaching has the advantage of immediate results while requiring less patient's effort
2). The recent introduction of resin barriers and commercially available bleaching systems with a highly concentrated hydrogen peroxide has also significantly improved the convenience of the process.
According to Goldstein
3), there are several factors that affect the effectiveness of tooth whitening. In addition to the cleanliness of the tooth surface and concentration of the hydrogen peroxide, temperature, pH, and the time duration, creating a sealed environment influences the effectiveness. A sealed environment is established during conventional home bleaching and walking bleach technique. In order to improve the effectiveness of in-office bleaching, a sealed environment should be considered.
A protocol of creating a sealed environment during in-office bleaching called "compressive bleaching technique"has been introduced by Miara
4), with claims of increased efficacy. In this technique, power bleaching gel is applied in a bleaching tray and the borders are sealed by a light-cured resin barrier. Power bleaching is thought to work by the permeation of oxygenating perhydroxyl free radicals through enamel micropores along a diffusion gradient and into the dentine where it oxidizes stains and bleaches the teeth
5,
6). It has been suggested that the compressive pressure lead to the penetration of oxygen radicals into the enamel. However, this technique needs a customized bleaching tray and the effectiveness hasn't been evaluated, yet.
Recently Kwon
7) introduced a new protocol of in-office bleaching called "sealed bleaching technique", which prevents the evaporation and dry-out of active agents by placing a linear low density polyethylene (LLDPE) wrap onto the power whitening gel. By creating a sealed environment around the bleaching gel, the activated bleaching agent remains concentrated near the tooth surface so that it is directed into the tooth rather than evaporating into air. This technique not only improves the effectiveness of bleaching but also makes it safer. Sealing prevents the bleaching agent from evaporating and reduces unintentional exposure. The activated bleaching agent is also utilized more effectively, and replenishment of bleaching agent is not necessary, making the procedure simpler. The whole in-office bleaching procedure may be completed without interruption for replenishment. Another advantage of sealed bleaching is that it does not need any additional lab procedure and may be used with any bleaching system regardless of light activation.
The "sealed bleaching technique"is a relatively new technique, and there hasn't been any in-vitro or clinical study evaluating this method. The purpose of this clinical study was to evaluate the effectiveness of sealing technique on in-office bleaching by comparing the outcome of sealed and conventional bleaching procedure.
II. MATERIALS & METHODS
The protocol for this study and the informed consent form were approved by the institutional review board of Yongdong Severance Hospital, Seoul, Korea.
Subjects
The upper anterior teeth of volunteers were examined clinically and radiographically. The patients who met the following criteria were selected to participate in this study (
Figure 1).
After the screening process, 2 men and 8 women qualified to participate in this study. Written informed consent was obtained from all patients.
Materials
- Bleaching agents and light
The bleaching agent used in this study was 1 scoop (0.4 ml) of Brite powder (PacDent, Walnut, CA, USA) mixed with 10 drops (0.4 ml) of 3% hydrogen peroxide and 0.4 ml of 15% carbamide peroxide gel (KoolWhite, PacDent, Walnut, CA, USA). Brite powder is a mixture of silica powder and light catalyst.
For light-activation, BT Cool light (APOZA, Taipei Hsien, Taiwan R.O.C), a high flux blue LED light unit with a wavelength of 430 - 490 nm was used.
Methods
- Shade evaluation
Color evaluation was performed independently using the following two methods at baseline, immediately after the bleaching procedure, and at the one-week follow-up appointment.
(1) Shade guide matching by an independent shade evaluator under natural daylight using the 16 shade tabs of Vita shade guide (Vita, Bad Sackingen, Germany) arranged by value order from lightest to darkest (
Table 1)
8). All shade guide matching was done by the same shade evaluator under the same condition. For each tooth, shades were assessed for the incisal, middle, and cervical 1/3 portion of the tooth.
(2) Color measurements using a spectrophotometer (Spectroshade, MHT, Niederhasli, Switzerland) by the spectrophotometer taker. To ensure accurate measurement, the unit was calibrated each time before use, and only images that were accurately taken, indicated by the green light on the screen, were used. ΔE from the Commission Internationale de l'Eclairage (CIE) L
*a
*b
* color system was used to determine the color difference between shades
8). ΔE is the shortest distance in the CIE L
*a
*b
* color space as determined by the following equation:
ΔE value was obtained using the Spectroshade analysis software (Ver. 2.41, MHT, Niederhasli, Switzerland).
-Study design
Each participant received thorough oral prophylaxis and polishing at least 1 week prior to bleaching.
The schematic diagram of the experimental procedure is shown in
Figure 2.
At the bleaching appointment, the preoperative baseline shade assessment of the upper anterior teeth was made. Initial photographs were taken at centric occlusion and at edge-to-edge bite using a 35-mm camera (Nikon F-801, Nikon, Tokyo, Japan; Kodak Professional Ektachrome transparency film E100, Kodak, Rochester, NY, USA).
A split-mouth design was used in this study. One side was randomly designated by the operator as the sealed side, and the contra-lateral teeth were used as control. This information was not shared with others.
To protect the soft tissue, OptraGate (Ivoclar-Vivadent, Schaan, Liechtenstein), cotton rolls, and gauze were used. A resin barrier (Kooldam, PulpDent, Watertown, MA, USA) was applied for gingival isolation and light cured. Patients were given protective eye goggles. The bleaching agent was applied to the labial surface of the upper anterior teeth. A 45 mm × 15 mm LLDPE wrap was applied as a tooth whitening cover to the 3 upper anterior teeth of the sealed side. The bleaching agent was light-activated with BT Cool light for 1 hour. Afterwards, bleaching agent was removed and the teeth were thoroughly rinsed with water.
A neutral fluoride gel (pH 7 neutral gel, Pascal, Bellevue, WA, USA) was applied to the labial side of the upper anterior teeth for 5 minutes. After waiting another 5 minutes for rehydration, postoperative evaluation was performed.
One week after the bleaching appointment, the patients were recalled for 1 week check-up. Shade assessments were made and photographs were taken. Patients were also asked to report any kind of sensitivity experienced during and after treatment.
Statistical analysis
The results of subjective shade guide matching were converted to numerical shade scores using
Table 1.
Paired t-test was used to compare the average shade score between the sealed side and control side before bleaching, after bleaching, and at check-up using S.A.S. Ver. 8.2 (SAS Institute, Inc., Cary, NC, USA). The average ΔE values were analyzed by paired t-test using Microsoft Excel 2003 software (Microsoft, Redmond, WA, USA) for paired t-test.
III. RESULTS
The average shade scores obtained from visual shade matching of each group at three different times were calculated, and are represented in
Table 2.
In the control and sealed groups, the average score in shade guide units after bleaching treatment showed statistically significant difference from the preoperative shade score (control group, p < .05; and sealed group, p < .05). In addition, the average shade scores at check up appointment also showed significant difference from the preoperative score (control group, p < .05; and sealed group, p < .05) (
Table 3).
Both the postoperative shade scores (p < .05) and the check up shade scores (p < .05) showed statistically significant difference between the control and sealed groups while there was no significant difference before the bleaching procedure (p > .05) (
Table 4).
The average ΔE values of each group were calculated and are shown in
Table 5. Compared to prebleaching status, the ΔE values at postbleaching condition were 4.35 ± 1.38 and 5.08 ± 1.34 for the control and sealed groups, respectively. The ΔE values at check up were 3.73 ± 1.95 and 4.38 ± 2.08 for the control and sealed groups. Paired t-test revealed that there were statistically significant differences in ΔE value between the control and sealed groups immediately after the bleaching procedure (p < .05) and at 1 week check up (p < .05).
IV. DISCUSSION
This study was designed to evaluate the effectiveness of in-office sealed bleaching technique compared to conventional in-office bleaching. In the clinical application, sealed bleaching technique had some advantages. First, according to the results of this study sealed bleaching was more effective than conventional technique for whitening the teeth. The shade improvement was greater on the sealed side. Second, as the sealed side was covered by LLDPE wrap, the bleaching agent remained moist until the completion of the bleaching procedure while the control side was relatively dry thus the evaporation of active agents into the air rather than into the tooth. Third, the exposure of the patient, dentist and dental staff to the evaporated bleaching agent can be minimized. The wrap prevents contact of the evaporated bleaching agent with any tissue. Fourth, since sealed bleaching technique allows for more effective use of the bleaching agent, lower concentration hydrogen peroxide may be used to produce the same bleaching result as higher concentration of hydrogen peroxide.
There is a general trend in seeking methods to effectively whiten teeth while using lower concentration hydrogen peroxide. The hydrogen peroxide concentration of the bleaching gel used in this study was about 3 ~ 4%. This may seem low compared to the various studies done in the past using higher concentrations of office bleaching agents
2,
9,
10). However, a controversy over the safety of using highly concentrated hydrogen peroxide still remains
6,
11) and in some countries, it is prohibited by law. Higher concentrations of hydrogen peroxide produce quicker whitening results
3), but it may also produce more cases of thermal sensitivity
12). With regard to sensitivity, during the in-office bleaching procedure one patient reported a slight sensitivity on the canine of the control side, which disappeared after 5 minutes. None of the patients reported any sensitivity during the one week follow-up. This may be attributed to the low concentration of hydrogen peroxide used. However, despite the low concentration of hydrogen peroxide, in both the control group and sealed group, there were significant shade changes after the bleaching treatment, and the shade changes were greater for the sealed group.
There were statistically significant differences in average shade scores or ΔE values between the groups, but the difference between the groups was less than the human perception threshold of 4 E units
7,
8). This is probably due to the fact that low concentration of hydrogen peroxide was used for only one session. In clinical settings usually multiple visits are required for in-office bleaching procedures, and the cumulative effects may add up to increased effectiveness of sealed bleaching. Further studies using different concentrations of hydrogen peroxide and multiple visits are necessary to verify the cumulative effects. Moreover, the quantity of oxygen radical that permeates into enamel with or without sealing needs to be determined, and its correlation with hypersensitivity needs to be investigated.
Several factors were considered in designing this study in order to objectively assess the effects of sealed bleaching. First of all, a split-arch design was used to keep variables as constant as possible
13). One of the most significant variables in the evaluations of in-office bleaching systems is the human test subject because of the difference in genetics and/or the inherent causes of the tooth discoloration. Moreover, a double blind protocol was used to ensure that the shade assessments were not biased. Since the patients were also blind to this information, the patients were asked if there was any difference in sensitivity.
The shade assessments were made using two methods to complement one another. First, visual color assessments were made using a value-oriented shade guide. Although it has been shown that the Vita Classic shade tabs are not systemically distributed in the color space relevant to the human teeth and that there is even over lapping
14), visual color assessment may be the most clinically relevant and significant since it most closely matches the patient's perspective
15).
Spectrophotometer was also used as an additional method. It is highly precise and accurate while being relatively easy to use. This instrument generates a spectral curve indicating the exact color of the tooth. The evaluation isn't influenced by illumination, metamerism, or simultaneous contrast effects as human visual perception
15). ΔE values were taken from the middle of each tooth because it is the area where spectrophotometer reading is the most accurate. The incisal third of the tooth is affected by the translucency of the tooth, and the two dimensional information obtained from the spectrophotometer might not be accurate enough
16). In addition, spectrophotometers are designed to evaluate flat surfaces, and the curved area of the cervical third area may offer less accurate readings
15). Therefore, while three shade scores of the cervical, middle, and incisal portions were obtained from each tooth for visual shade assessment, spectrophotometer value was taken from only the middle area of each tooth at each time.
Although two different methods were used to evaluate the tooth shade change, both methods consistently showed that sealed bleaching was more effective than conventional in-office bleaching. In comparison of shade score change, the sealed group showed significantly greater shade change than the conventional bleaching group. In addition, the sealed group also showed significantly greater shade change in terms of ΔE value.
Paired t-test was used for statistical analysis since the contra-lateral teeth of the same subject were being compared. For spectrophotometer readings, postoperative and check up ΔE values were used for comparison since E value can be considered as a uniform unit of measurement evenly distributed in color space. However, the changes in shade scores could not be compared directly between groups since the shade tabs are not evenly distributed. Therefore, the shade scores at the time of each measurement were compared directly between groups.
Tooth dehydration is a probable cause of immediate tooth lightening
10). It also takes time for oxygen to be released completely and not interfere with the optical properties of the tooth structure
9,
17). Therefore, an immediate postoperative shade taking may not accurately represent the shade change. On the other hand, as the follow-up period increases, the relapse of the bleaching effect is affected by the habits of the patient such as smoking or coffee drinking. Therefore, in order to limit this study to evaluating the effect of sealed bleaching, a one-week follow-up period was used.
Sealed bleaching technique was introduced as a way to improve on the effectiveness, safety and convenience of in-office bleaching procedure, and this study investigated the effectiveness of sealed bleaching technique in a randomized clinical trial. The results of this study demonstrate that sealed bleaching increased the effectiveness of in-office bleaching procedure used in this study. This innovative technique may be used to improve the efficacy of power bleaching without increasing the concentration of hydrogen peroxide used.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


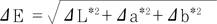







 XML Download
XML Download