Abstract
The purinoreceptor, P2X3 is a ligand-gated cation channel activated by extracellular ATP. It has been reported that ATP can be released during inflammation and tissue damage, which in turn may activate P2X3 receptors to initiate nociceptive signals. However, little is known about the contribution of P2X3 to the dental pain during pulpal inflammation. Therefore, the purpose of this study was to investigate the expression of P2X3 and its colocalization with TRPV1 to understand the mechanism of pain transmission through P2X3 in the human dental pulp with double labeling immunofluorescence method.
In the human dental pulp, intense P2X3 immunoreactivity was observed throughout the coronal and radicular pulp. Of all P2X3-positive fibers examined, 79.4% coexpressed TRPV1.
This result suggests that P2X3 along with TRPV1 may be involved in the transmission of pain and potentiation of noxious stimuli during pulpal inflammation.
Nociceptors express ion channels and receptors that respond to noxious stimuli and so are involved in the initiation of pain. Among several receptors, ATP purinoreceptor P2X3 and Vanilloid receptor TRPV1 are 2 key molecules in pain mechanism1,2).
P2X3 receptor is one of the many ligand-gated ion channels and is activated by extracellular ATP3). To date, 7 P2X receptor subunits have been identified by molecular cloning. In particular, attention has focused on P2X3 receptors because they are expressed selectively in a subset of sensory neurons which are potentially nociceptors4,5). ATP is well recognized as an energy source and a modulator of cellular function. In the nervous system, ATP acts as a neurotransmitter and serves as a mediator of pain through binding to P2X3 receptor. ATP can be released actively or passively by cell lysis during tissue damage, which in turn may activate P2X3 receptors to initiate nociceptive signals. This effect may be exaggerated under conditions of inflammation6,7).
Many previous studies showed the expression of P2X3 in the central and peripheral neurons1,4,8-10). In the orofacial region, it has been reported that P2X3 immunoreactivity exists in dental pulp3), taste bud8), and temporomandibular joint11) of the rat. The presence of P2X3 in the human dental pulp was also documented in some studies12,13). The presence of P2X3 in fibers of human dental pulp suggested that they may play a role in the perception of dental pain.
The vanilloid receptor TRPV1 is a marker of nociceptive primary afferent neurons. TRPV1 has been suggested as a molecular integrator of chemical and physical stimuli that elicit pain14). Functionally, TRPV1 is essential for the development and maintenance of thermal hyperalgesia and allodynia, which are associated with inflammation and tissue injury15). Previous studies showed the expression and potential role of TRPV1 in the human dental pulp12,16,17). It was suggested that TRPV1 may play an important role in neurogenic inflammation and pain transmission17) and involved in inflammatory hyperalgesia and thermal nociception in the human dental pulp16).
Based on the contribution of P2X3 and TRPV1 to pain mechanism, several studies were performed to investigate the coexpression of P2X3 and TRPV1. However, they showed variable results. At the microscopic level, it was reported that many P2X3-positive cells coexpress TRPV1 in the dorsal root ganglion and trigeminal ganglion of the rat18), In this study, 75% of P2X3-positive neuron was costained with TRPV1 in the dorsal root ganglion, and 96% in the trigeminal ganglion. This result is inconsistent with that of other study showing that 35% and 9% of P2X3-positive neuron, respectively in the trigeminal ganglion and dental pulp of the rat, were costained with TRPV119). It seems that a surprising feature of this result is that the coexpression of P2X3 and TRPV1 is rare in the dental pulp, peripheral terminal of trigeminal sensory system, while frequent in the trigeminal ganglion. It is also questionable that this result can be applicable to the human dental pulp. The coexpression pattern of P2X3 and TRPV1 in the human dental pulp is still unknown.
Therefore, the aim of this study was to investigate the colocalization of P2X3 and TRPV1 in the human dental pulp with double labeling immuno-fluorescence method.
Human first or second premolars without caries, restorations or periodontal disease that had been extracted for orthodontic treatment were used in this study (n = 10). Subsequent to the extraction process within a time lapse of 1 hour, the teeth were split longitudinally with a water-cooled high speed diamond bur to take out pulp tissues. The tissues were post-fixed for 2 hours with 4% (w/v) paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, and were cryoprotected in 30% sucrose in 0.1 M phosphate buffer, pH 7.4 overnight at 4℃. On the following day, cryoprotected pulp tissues were frozen on dry ice and 60 µm-thick sections were cut on a freezing microtome and the sections were immersed in 0.1 M phosphate buffer, pH 7.4.
All incubations for light microscopic immunocytochemistry were carried out on a shaker at room temperature. Sections of the tooth pulp were permeabilized with 50% ethanol for 30 min, blocked with 10% normal donkey serum for 30 min (Jackson Immunoresearch, West Grove, PA, USA) to mask secondary antibody binding site, and incubated overnight in primary antibodies in phosphate-buffered saline (0.01 M, pH 7.2). We used the following primary antibodies in double combinations: anti-P2X3 (raised in rabbit; 1 : 7,000; Neuromics, Northfield, MN, USA), anti-TRPV1 (raised in goat; 1 : 700; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After several rinses in phosphate-buffered saline (0.01 M, pH 7.2) and distilled water, and incubation with 2% normal donkey serum for 30 min, sections were transferred to a mixture of the secondary antibodies containing Cy3-conjugated anti-rabbit, fluorescence isothiocyanate-conjugated anti-goat (1 : 200; Jackson immunoresearch, West Grove, PA, USA) for 3 hr. After several rinses, sections were mounted on slides and dried in 50℃ oven for 10 min. Sections were coverslipped with Vectashield (Vecta laboratories, Burlingame, CA, USA), and were examined on a confocal microscope (LSM 510 Meta, Carl Zeiss Inc., Germany). Confocal images were saved in TIFF format, contrast and brightness were adjusted, and final plates were composed using Photoshop software (version 7.0, Adobe Systems, San Jose, CA, USA). Control sections were processed as described above, except that primary or secondary antibodies were omitted or replaced by control antisera. Omission of primary or secondary antibodies eliminated a specific staining.
Colocalization of P2X3 and TRPV1 was quantitatively analyzed. P2X3 and TRPV1 immunoreactivites in nerve fibers were examined and quantified by computerized image analysis (i-solution, iMTechnology, Daejeon, Korea). Counts of P2X3 immunopositive fibers were taken from the random fields (100 × 100 µm2 in each field) of two or three areas in each section. In every image, the number of P2X3-positive fibers showing colocalization with TRPV1 was counted. Results were expressed as percentages of TRPV1-positive fibers to P2X3-positive fibers in given areas. Inter-teeth variability in the colocalization was insignificant and thus the data were pooled.
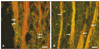
P2X3-positive fibers were identified in the human dental pulp. Intense P2X3 immunoreactivity was observed throughout the pulp. The distribution of P2X3 immunoreactive nerve fibers in the human dental pulp is shown in Figure 1. There was no apparent difference in the expression of P2X3 in the central and peripheral portion of the pulp. Immunoreactive nerves were seen traveling in bundles throughout the bulk of the tissues (Figure 1B), sometimes accompanying blood vessels (Figure 1A).
Double immunofluorescence revealed that P2X3-positive fibers were frequently costained with TRPV1 (Figure 2). There was no apparent difference between the central and peripheral portion of the pulp in the colocalization of P2X3 with TRPV1. Of a total of 199 P2X3-positive fibers examined, 79.4% (158/199) coexpressed TRPV1. Only 20.6% (41/199) did not coexpress TRPV1. Figure 3 shows confocal micrographs presenting the nerve fibers stained for P2X3 only.
This study demonstrated that P2X3 immunoreactivity is present in the human dental pulp and numerous P2X3-positive nerve fibers coexpress TRPV1.
Alavi et al.12) investigated the levels of the P2X3 in the human dental pulp. They showed that intense P2X3 immunoreactivity was observed in main body of the pulp, in the subodontoblastic plexus of Raschkow, and within the odontoblastic area. This observation is consistent with our finding showing Intense P2X3 immunoreactivity throughout the pulp. However, Renton et al.13) reported that P2X3 immunoreactive fibers were found throughout the pulp and the expression was weak. They attributed the differences of results to experimental design. They used peripheral subodotoblastic layer instead of densely innervated central area and that might account for some differences with the other results.
P2X3 is expressed primarily in nociceptive afferent nerve terminals4,5) and they have small to medium-sized cell bodies in sensory ganglia18-20). Earlier study demonstrated that P2X3 receptors are present on both myelinated (Aδ) and unmyelinated (C) nerve fibers in the human dental pulp and may play a role in dental pain mechanism12). Lesion study showed that P2X3 immunoreactivity traffics both centrally and peripherally. As for the peripheral projections, P2X3 immunoreactivity has been reported in fine unmyelinated fibers in tongue, viscera, tooth pulp and skin21). In the present study, P2X3-positive fibers were identified in the human dental pulp and intense P2X3 immunoreactivity was observed throughout the pulp. This result is consistent with that of previous study by Renton et al.12) and may indicate the possible role of P2X3 in the human dental pulp.
In many previous studies on the expression of P2X3, normal tissues without inflammation were used and the role of P2X3 was inferred from the results of the studies. It was also reported that the expression of P2X3 is upregulated in the pathologic condition22,23). Thus, we used the healthy pulp tissue with the idea that the expression of P2X3 itself in the healthy pulp would indicate the involvement of P2X3 in pain transmission. And we found that it was very difficult to get proper stage of inflammation we want through the pilot study.
In the present study, majority of P2X3-positive nerve fibers also expressed TRPV1. However, in the previous study using temporomandibular joint of the rat, coexpression of P2X3 and TRPV1 was abundant in facial skin and relatively rare (6%) in tooth pulp neuron19). In the trigeminal ganglion of the rat, a large number of P2X3-positive neuron (96%) also expressed TRPV1 and 92.9% of the P2X3-positive fibers were unmyelinated and 7.1% were myelinated in the sensory root just proximal to the trigeminal ganglion20). This discrepancy of coexpression pattern may reflect species difference (rat vs. human) and difference between peripheral and central distribution of these markers.
It is important that how we can apply these results to explain the mechanism of pain transmission. In our previous studies, we demonstrated that TRPV1 is expressed in human dental pulp and may be involved in pain transmission during pulpal inflammation16,17). On the ultrastructure of TRPV1-positive nerve terminals in the human dental pulp, there were two types of TRPV1 immunoreactive nerve fibers identified: one containing clear round vesicles and many densecored vesicles, the other containing clear round vesicles and few dense-cored vesicles. It is known that dense-cored vesicles contain neuropeptides such as substance P and calcitonin gene-related peptide, and clear round vesicles contain neurotransmitter such as glutamate24-26). In the previous study using human dental pulp, 40% of P2X3-positive nerve fibers were IB4-binding and nonpeptidergic12). Moreover, P2X3-positive nerve fibers and terminals in the trigeminal sensory nuclei contained many clear, round, synaptic vesicles with few dense-cored vesicle27). From the described above, it is assumed that P2X3 may present in the TRPV1-positive nerve fiber containing clear round vesicles and involved in pain transmission with similar mechanism of nonpeptidergic TRPV1. When the stimulus is present, glutamate which is released from nerve ending binds glutamate receptor such as α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA), N-methyl-D-aspartate (NMDA) receptor of adjacent nerve fibers. Consequently, it is thought that noxious stimulus is potentiated by release of glutamate from adjacent nerve ending simultaneously with transmission of stimulus centrally. On the other hand, peptidergic TRPV1 may be involved in sustained pain and hyperalgesia during pulpal inflammation. TRPV1-positive pulpal fibers activated by inflammatory mediators may release neuropeptides from their terminals through the axon reflex28). These neuropeptides induce vasodilation and increased capillary permeability, which in turn induce the release of inflammatory mediators such as bradykinin, histamine, prostaglandin E29). These cyclic processes may attribute to sustained pain and hyperalgesia during pulpal inflammation.
However, there are accumulating evidences demonstrating the direct involvement of P2X3 in hyperalgesia and allodynia. Studies with antagonists selective for P2X3-containing receptor showed marked reduction in chronic inflammation induced by thermal30) and mechanical31) hyperalgesia, and spinal nerve ligation-induced mechanical allodynia30). Moreover, it has been reported that nociceptive action of ATP are markedly augmented in the presence of inflammation or inflammatory mediators. Paukert et al.32) showed that inflammatory mediator such as substance P and bradykinin potentiate the ATP-evoked currents in oocytes expressing P2X3 by lowering the desensitization rate through phosphorylation of P2X3. Hamilton et al.7) also reported that nociceptive action of ATP are markedly augmented in the presence of inflammation or inflammatory mediators and it was attributed that extracellular levels of ATP will reach levels capable of activating nociceptors in inflamed tissue. There are several ways in which the enhanced responses might arise. One possibility is changes in the pH of the extracellular environment, since there is ample evidence that pH can modify responses of P2X receptors when studied in culture. Inflammation can also be associated with tissue acidosis. Another possibility is that inflammatory stimuli might upregulate receptor levels in nociceptor. As a result, it is possible that P2X3 can be directly involved in hyperalgesia and allodynia during pulpal inflammation. Therefore, further studies are needed to elucidate the specific role played by P2X3 in the mediation of pain in the human dental pulp besides above explanation. In the present study, overall colocalization of P2X3 and TRPV1 was investigated. However, no study was performed on the identification of the types of P2X3 immunoreactive nerve fibers. The relative proportion of peptidergic and nonpeptidergic nerve fibers among P2X3 immunoreactive nerve fibers coexpressing TRPV1 and the ultrastructural characteristics of P2X3 immunoreactive nerve fiber through preembedding immunocytochemistry are also needed to elucidate the more detailed mechanism of pain transmission through P2X3 receptor in the human dental pulp.
Taken together, our findings suggest that the expression of P2X3 and frequent colocalization with TRPV1 in the human dental pulp may account for their important roles in pain transmission during pulpal inflammation.
Based on the results of this study, P2X3 immunoreactivity is present in the human dental pulp and numerous P2X3-positive nerve fibers coexpress TRPV1. It is suggested that P2X3 along with TRPV1 may be involved in the transmission of pain and potentiation of noxious stimuli during pulpal inflammation.
Figures and Tables
 | Figure 1Photomicrographs of P2X3 immunoreactive nerve fibers in the human dental pulp (× 200). A shows P2X3 immunoreactive nerve fibers (arrows) accompanying blood vessel (asterisks) in the coronal pulp, and B shows P2X3 immunoreactive nerve fibers (arrows) in the radicular pulp. Scale bars in A and B = 50 µm. |
References
1. Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoreceptor expressed by a subset of sensory neurons. Nature. 1995. 377(6548):428–431.

2. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levin JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997. 389(6653):816–824.

3. Cook SP, Vulchanova L, Hargreaves KM, Wide R, McClesky EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997. 387(6632):505–508.

4. Dunn PM, Zhing Y, Burnstock G. P2X receptors in peripheral neurons. Prog neurobiol. 2001. 65:107–134.

5. North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000. 40:563–580.

6. Ding Y, Cesare P, Drew L, Nikitaki D, Wood JN. ATP, P2X receptors and pain pathway. J Auton Nerv Syst. 2000. 81:289–294.
7. Hamilton SG, Wade A, McMahon SB. The effect of inflammation and inflammatory mediators on nociceptive behaviour induced by ATP analogues in the rat. Br J Pharmacol. 1999. 126:326–332.
8. Bo X, Alavi A, Xiang Z, Oglesby I, Ford A, Burnstock G. Localization of ATP-gated P2X2 and P2X3 receptor immunoreactive nerves in rat taste buds. Neuroreport. 1999. 10(5):1107–1111.
9. North RA. P2X3 receptors and peripheral pain mechanism. J Physiol. 2004. 554(Pt 2):301–308.
10. Llewellyn-Smith IJ, Burnstock G. Ultrastructural localization of P2X3 receptors in rat sensory neuron. Neuroreport. 1998. 9:2545–2550.

11. Ichikawa H, Fukunaga T, Jin HW, Fujita M, Takana-Yamamoto M, Sugimoto T. VR1- and VRL-1- and P2X3 receptor-immunoreactive innervation of the rat temporomandibular joint. Brain Res. 2004. 1008(1):131–136.
12. Alavi AM, Dubyak GR, Burnstock G. Immunohistochemical evidence for ATP receptors in human dental pulp. J Dent Res. 2001. 80(2):476–483.

13. Renton T, Yiangou Y, Baecker PA, Ford AP, Anand P. Capsaicin receptor VR1 and ATP Purinoceptor P2X3 in painful and nonpainful human tooth pulp. J Orofac Pain. 2003. 17:245–250.
14. Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998. 21:531–543.

15. Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harris MH, Latcham J, Clapham C, Atkinson K, Hugfes SA, Rance K, Grau E, Harper AJ, Pugf PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000. 405:183–187.

16. Kim YK, Ma SK, Jin MU, Kim SK, Bae YC. The ultrastructure of TRPV1-positive nerve terminals in the human tooth pulp. Korean J Anat. 2006. 39(4):297–303.
17. Kim YK, Kim SK. Immunocytochemical study on the expression of TRPV1 in the human dental pulp. J Korean Acad Endod. 2007. 8(1):79–87.
18. Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoreceptor and IB4 binding sites. Eur J Neurosci. 1999. 11:946–958.

19. Ichikawa H, Sugimoto T. The co-expression of P2X3 receptors with VR1 and VRL-1 in the rat trigeminal ganglion. Brain Res. 2004. 998:130–135.

20. Kim YS, Paik SK, Cho YS, Shin HS, Bae JY, Moritani M, Yoshida A, Ahn DK, Valtschanoff J, Hwang SJ, Moon C, Bae YC. Expression of P2X3 receptor in the trigeminal sensory nuclei of the rat. J Comp Neurol. 2008. 506:627–639.

21. Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial derived neurotrophic factor. Mol Cell Neurosci. 1998. 12:256–268.

22. Brady CM, Apostolidis A, Yiangou Y, Baecker PA, Ford AP, Freeman A, Jacques TS, Fowler CJ, Anand P. P2X3-immunoreactive nerve fibers in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur Urol. 2004. 46:247–253.

23. Eriksson J, Bongenhielm U, Kidd E, Matthews B, Fried K. Distribution of P2X3 receptors in the rat trigeminal ganglion after inferior alveolar nerve injury. Neurosci lett. 1998. 254:37–40.

24. Bae YC, Ihn HJ, Park MJ, Ottersen OP, Moritani M, Yoshida A, Shigenaga Y. Identification of signal substances in synapses made between primary afferents and their associated axon terminals in the rat trigeminal sensory nuclei. J Comp Neurol. 2000. 418:299–309.

25. Johnson MD, Yee AG. Ultrastructure of electrophysiologically-characterized synapses formed by serotonergic raphe neurons in culture. Neuroscience. 1995. 67:609–623.

26. Tachibana M, Wenthold RJ, Morioka H, Petralia RS. Light and electron microscopic immunocytochemical localizaation of AMPA-selective glutamate receptors in the rat spinal cord. J Comp Neurol. 1994. 344:431–454.

27. Jin YH, bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and Vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitatius. J Neurosci. 2004. 24(20):4709–4717.

28. Kim S. Neurovascular interaction in the dental pulp in health and inflammation. J Endod. 1990. 16:48–53.

30. Barclay J, Patel S, Dorn G, Wotherspoon G, Moffatt S, Eunson L, Abdel'al S, Natt F, Hall J, Winter J, Bevans S, Wishart W, Fox A, Ganju P. Functional downregulation of P2X3 receptor subunit in rat sensory neurons reveals a significant role in chronic neuropathic and inflammatory pain. J Neurosci. 2002. 22(18):8139–8147.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download