Abstract
This report describes clinical cases of a palato-gingival groove on a maxillary lateral incisor with associated localized periodontal disease and pulp necrosis. The tooth of the first case was extracted because of severe bone destruction. The palato-gingival groove of the second case was eliminated using a round bur, and the resulting defect was filled with synthetic graft and covered by an absorbable membrane. Both diagnosis and treatment of palato-gingival groove were very difficult and usually extraction of the involved tooth is the treatment of choice, but combined endodontic-periodontic treatment allowed the tooth to be saved.
The palato-gingival groove is defined as "a developmental groove in a root that, when present, is usually found on the lingual aspect of maxillary incisor teeth"1). The formation of palato-gingival groove is probably caused by infolding of the enamel organ and the epithelial sheath of Hertwig during odontogenesis2) and has been speculated to be an aborted formation of an additional root3). Ennes and Lara suggested that the palatal groove could be the result of an alteration of genetic mechanisms4).
The anomaly has been termed the radicular anomaly3), distolingual groove5), radicular lingual groove6), palatoradicular groove7), radicular groove8) and cinguloradicular groove9). As the name implies, this malformation is actually a groove, which starts near the cingulum of the tooth and runs towards the cementoenamel junction in an apical direction at various depths and distances along the root surface.
Palato-gingival grooves on maxillary incisors often present a diagnostic and treatment-planning dilemma. While frequently associated with periodontal pockets and bone loss, pulpal necrosis of these teeth may precipitate a combined endodontic-periodontic lesion. This paper describes two cases involving maxillary lateral incisors with a deep palato-gingival groove and associated periodontal and pulpal involvement.
A 31-year-old male presented complaining of a purulent discharge from his gingiva and bad mouth odor. The patient was unaware of any previous trauma to the maxillary anterior region. Clinical examination revealed a draining sinus tract on the labial gingival surface associated with the maxillary right lateral incisor (Figure 1A). Thermal and electric pulp vitality tests were negative. The patient had no significant tenderness to percussion or palpation in the maxillary anterior area and probing depths were normal, except for a narrow 8 mm pocket on the palatal aspect (Figure 1B). Diagnostic radiographs revealed an extensive periradicular radiolucency associated with the affected tooth, and the facial sinus tract was traceable with a gutta-percha cone to the distal area of maxillary right lateral incisor (Figure 1C). In addition to a main pulp space in the tooth, a narrow vertically oriented radiolucent line was also evident on the radiograph. According to the tests and the radiographic findings, diagnosis was pulp necrosis with suppurative apical periodontitis and localized periodontitis secondary to the palatal groove on maxillary right lateral incisor.
Endodontic therapy was completed in three sessions using a 5.25% sodium hypochlorite solution as an irrigant, and calcium hydroxide (Pulpdent; Pulpdent Co., Watertown, MA, USA) as an 8-week interappointment canal dressing. Root canal obturation was completed with gutta-percha (Diadent, Burnaby, BC, Canada) and AH-26 sealer (Dentsply, Tulsa, OK, USA). The access cavity was then temporary sealed with Caviton (GC, Tokyo, Japan).
A week later, however, the patient presented with localized swelling around the affected tooth. An exploratory surgery was indicated and the patient was advised that the prognosis for the tooth may be hopeless. After elevation of the flap, a narrow palatal bony defect was noted extending 10 mm from the adjacent bony crest. This defect, which coursed along the palato-gingival groove, wrapped around to the facial and communicated with a facial bony destruction of the cortical plate (Figure 2A and B). Since the prognosis of the tooth was poor from a conservative standpoint, due primarily to severe bone destruction, the tooth was extracted. The examination of the extracted tooth revealed a palato-gingival groove extending from the cingulum to the apical portion (Figure 2C).
A 65-year-old male presented with discomfort in the region of maxillary right lateral incisor. Relevant medical history included a valvular disease of the heart. There was no history of trauma or injurious habit to his teeth. Intraoral examination revealed a sinus tract located on the buccal gingiva and a discoloration of crown of that tooth. The tooth did not respond to electric pulp test and had mobility. The patient described pain on percussion and a probing depth was 5 mm only along the palato-gingival groove. Radiographic examination revealed three vertically oriented radiolucent lines extending to the apex of the root. Based on the tests and the radiographic findings, the diagnosis was necrotic pulp and suppurative periradicular periodontitis.
The tooth was accessed and two root canals were identified using microscope and ultrasonic instruments. The canals were cleaned and shaped using stainless steel hand files (Dentsply-Maillefer, Ballaigues, Switzerland) and Ni-Ti rotary instruments (Protaper, Dentsply-Maillefer, Ballaigues, Switzerland), irrigated with 5.25% sodium hypochlorite. Despite of irrigation and calcium hydroxide dressing several times, the facial sinus tract and the patient's symptom were still present. Therefore, surgical treatment was suggested.
Based on the study of Schwartz et al10), the apical one-third of each canal was obturated with gutta-percha (Diadent, Burnaby, BC, Canada) and sealer (AH26; Dentsply, Tulsa, OK, USA). In anticipation of the need for significant odontoplasty of the palato-gingival groove, dual-cure composite resin (Luxacore; DMG, Hamburg, Germany) was then placed into middle and cervical thirds of the two canals for the best possible seal of the root canal system. Finally the access cavity was filled with light cure composite resin (Filtek Z-350; 3M, Saint Paul, MN, USA) (Figure 3C).
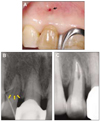
Under 2% lidocaine with 1 : 100,000 epinephrine local anesthesia, a full thickness mucoperiosteal flap utilizing intrasulcular incision was raised on both buccal and palatal sides. A bony defect extended to middle portion of the root along the palato-gingival groove and communicated with the facial fenestration (Figure 4A and B). The pathological granulation tissues were removed and the root surface was planed with curettes. The groove was saucered out using a small round diamond bur and apicoectomy and retrograde filling with MTA (ProRoot; Dentsply, Tulsa, OK, USA) were performed. Then the root surfaces were treated with 17% EDTA (Pulpdent Co., Watertown, MA, USA), and the bony defect was filled with synthetic graft (Biocera; Oscotec, Cheonan, Korea) (Figure 4C). An absorbable membrane (Lyoplant; B.Braun, Tuttlingen, Germany) was placed over the graft, and the flaps were sutured. After one week, teeth were temporary splinted, as a continuous mobility degree 2, under consultation with periodontal department.
At 6-months recall, the sinus tract had closed and the patient was asymptomatic. Sign of periodontal healing were evident. 3 mm non-bleeding sulcus was present at the site of the palatal groove. Radiographically, the density of bony trabeculae had increased and periodontal ligament space in the mesial side of the lateral incisor became narrow (Figure 5). Tooth mobility was remarkably decreased, and then temporary resin-wire splint was removed.
The palato-gingival groove is a developmental anomaly of variable extent and depth that may or may not involve a communication between the pulp cavity and the periodontal tissue. The anomaly generally has a funnel-like shape, which forms a niche where bacterial plaque and calculus accumulate; this makes it difficult, and sometimes impossible, for the patient or even the professional to clean properly. Inflammation may thus develop in the periodontal tissue adjacent to the groove. While the epithelium is intact, the periodontium will probably remain healthy. Once the attachment of the junctional epithelium has been ruptured, however, an infrabony periodontal pocket will develop along the entire extension of the groove. Not all grooved teeth will present a breakdown of the epithelial attachment, but the presence of the anomaly constitutes a risk factor of which the dentist should be aware. Whithers et al.11) reported that the palato-gingival groove is associated with a "poor periodontal health." Unfortunately, the "poor periodontal health"combined with a deep groove may put the tooth at risk, with poor prognosis12), despite periodontal and endodontic treatment13-15).
Diagnosis of a palato-gingival groove is not always easy. This is because the defect may manifest itself with symptoms of true periodontal desease or may be expressed as a true endodontic problem, while in other cases, it may appear as a combined endodontic-periodontic problem. Differential diagnosis should include a crack on the crown or a vertical root fracture. However, vertical root fracture of a sound central incisor is rare. The final diagnosis is greatly aided by the detection of the notch in the tooth's crown. It should be pointed out that it is not easy to detect this notch as it may be hidden below the gingival margin or by plaque. Periodontal probing is recommended and deep isolated periodontal pocket is found out. Moreover, grooves may be visible on a periapical radiograph as one or more dark lines extending along the length of the root, parallel to or superimposed over the root canal; these have been termed "parapulpal lines"5). In the present cases, this line was observed in periapical radiographs, and this helped us with detecting these grooves.
The treatment of a palato-gingival groove presents a clinical challenge to the operator and must involve a multidisciplinary approach. The variability in size and shape of this anomaly coupled with bacterial invasion may affect both the periodontium and the pulp. In such cases, bacterial colonization of the groove will cause periodontal inflammation and subsequent tissue breakdown. Pulp involvement may result as a result of introduction of bacterial toxins via channels that exist between the root canal system and the groove. We observed the presence of this communication in cross-sectional view of extracted tooth (Figure 2D). Once the pulp becomes necrotic, endodontic therapy is indicated. However, conventional endodontic treatment alone will not be effective because the bacterial etiology is residing extraradicularly as a self-sustaining lesion.
The reported long-term prognosis of therapy appears to be mostly related to the apical extension of the groove. Shallow grooves may often be treated successfully, while deep grooves present complex endodontic-periodontal problems with poor prognosis. In the first case, the palato-gingival groove was deep and extended to the apical third of the root. Moreover, progression of periodontal disease had been sustained for long time and the destruction of periodontium was extensive subsequently.
These case reports involved maxillary lateral incisors with a deep palatal groove and associated periodontal and pulpal involvement. In the first case, as a result of the extensive nature of this groove on the lingual surface, the maxillary right lateral incisor had a severe localized periodontal disease requiring an extraction. In the second case, however, the groove was not extended deeply to apical portion. In this respect, the tooth was treated using a combined endodontic-periodontic treatment approach, and periodontal ligament attachment and periradicular healing were evident both clinically and radiographically at the 6-month follow-up.
Figures and Tables
Figure 1
Pretreatment images. (A) Photograph demonstrating the facial sinus tract. (B) Probing depth (8 mm) at the cingulum. (C) Periapical radiograph showing a gutta-percha cone tracing the facial sinus tract.
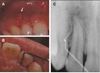
Figure 2
(A) and (B) The osseous defect associated with the palato-gingival groove (arrow). (C) Lingual side of extracted tooth shows palato-gingival groove running along entire length of root. (D) Cross sectional view of extracted tooth at 5 mm level from CEJ shows the communication between the root canal system and the groove.
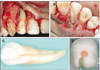
Figure 3
(A) Preoperative clinical view demonstrating the facial sinus tract (arrow) and discoloration of lateral incisor. (B) Periapical radiograph showing a gutta-percha cone tracing the facial sinus tract to the periradicular radiolucency associated with the right maxillary lateral incisor. Three vertically oriented radiolucent lines are also evident within lateral incisor (arrows). (C) Nonsurgical endodontic treatment; gutta-percha on apical one-third and resin core on middle and coronal one-third.
References
1. American Association of Endodontists. Glossary of endodontic terms. 2003. 7th ed. Chicago, IL:
2. Lee KW, Lee EC, Poon RY. Palato-gingival grooves in maxillary incisors. A possible predisposing factor to localized periodontal disease. Br Dent J. 1968. 124:14–18.
3. Simon JH, Glick DH, frank AL. Predictable endodontic and periodontic failure as a result of radicular anomalies. Oral Surg Oral Med Oral Pathol. 1971. 31:823–826.

4. Ennes JP, Lara VS. Comparative morphological analysis of the root developmental groove with the palate-gingivalgroove. Oral Dis. 2004. 10:378–382.

5. Everett FG, Kramer GM. The disto-lingual groove in the maxillary lateral incisor; a periodontal hazard. J Periodontol. 1972. 43:352–361.

6. August DS. The radicular lingual groove: an overlooked differential diagnosis. J Am Dent Assoc. 1978. 96:1037–1039.

7. Kogon SL. The prevalence, location and conformation of palato-radicular grooves in maxillary incisors. J Periodontol. 1986. 57:231–234.

8. Pecora JD, Sousa Neto MD, Santos TC, Saquy PC. In vitro study of the incidence of radicular grooves in maxillary incisors. Braz Dent J. 1991. 2:69–73.
9. Assaf ME, Roller N. The cingulo-radicular groove: its significance and management - two case report. Compendium. 1992. 13:94. 96. 98 passim.
10. Schwartz SA, Koch MA, Deas DE, Powell CA. Combined endodontic-periodontic treatment of a palatal groove: a case report. J Endod. 2006. 32:573–578.

11. Withers JA, Brunsvold MA, Killoy WJ, Rahe AJ. The relationship of palato-gingival grooves to localized periodontal disease. J Periodontol. 1981. 52:41–44.

12. Rosling B, Nyman S, Lindhe J. The effect of systematic plaque control on bone regeneration in infrabony pocket. J Clin Periodontol. 1976. 3:38–53.

13. Fabra Campos H, Millet Part J. Developmental radicular groove as a cause of endodontic failure. Rev Esp Endodoncia. 1989. 7:118–123.
14. Pack AR, Chandler NP. A combined endodontic-periodontal lesion of development origin: a case report. N Z Dent J. 1996. 92:46–48.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download