1. Kakehashi S, Stanley HR, Fizgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965. 20:340–349.

2. Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981. 89:321–328.

3. Haapasalo M, Endal U, Zandi H, Jeffrey M. Eradication of endodontic infection by instrumentation and irrigation solutions. Endod Topics. 2005. 10:77–102.

4. Sundqvist G, Fiigdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998. 85:86–93.

5. Heling I, Chandler NP. Antimicrobial effect of irrigant combinations within dentinal tubules. Int Endod J. 1998. 31:8–14.

6. Barrette WC Jr, Hannum DM, Wheeler WD, Hurst JK. General mechanism for the bacterial toxicity of hypochlorous acid: abolition of ATP production. Biochemistry. 1989. 28:9172–9178.

7. McKenna SM, Davies KJ. The inhibition of bacterial growth by hypochlorous acid. Possible role in the bactericidal activity of phagocytes. Biochem J. 1988. 254:685–692.

8. Siqueira JF Jr, Machado AG, Silveira RM, Lopes HP, de Uzeda M. Evaluation of the effectiveness of sodium hypochlorite used with three irrigation methods in the elimination of Enterococcus faecalis from the root canal,
in vitro. Int Endod J. 1997. 30:279–282.

9. McComb D, Smith DC, Beagrie GS. The results of
in vivo endodontic chemomechanical instrumentation--a scanning electron microscopic study. J Br Endod Soc. 1976. 9:11–18.

10. Coates D. Comparison of sodium hypochlorite and sodium dichloroisocyanurate disinfectants: neutralization by serum. J Hosp Infect. 1988. 11:60–67.

11. Coates D. A comparison of sodium hypochlorite and sodium dichloroisocyanurate products. J Hosp Infect. 1985. 6:31–40.

12. Russell AD, Day MJ. Antibacterial activity of chlorhexidine. J Hosp Infect. 1993. 25:229–238.

13. White RR, Hays GL, Janer LR. Residual antimicrobial activity after canal irrigation with chlorhexidine. J Endod. 1997. 23:229–231.

14. Russell AD. Activity of biocides against mycobacteria. Soc Appl Bacteriol Symp Ser. 1996. 25:87S–101S.

15. Goldman M, Pearson AH. Postdebridement bacterial flora and antibiotic sensitivity. Oral Surg Oral Med Oral Pathol. 1969. 28:897–905.
16. Basrani B, Tjaderhane L, Santos JM, Pascon E, Grad H, Lawrence HP, Friedman S. Efficacy of chlorhexidine- and calcium hydroxide-containing medicaments against Enterococcus faecalis
in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003. 96:618–624.

17. Haapasalo M, Østavik D. In vitro Infection and Disinfection of Dentinal Tubules. J Dent Res. 1987. 66:1375–1379.
18. Eddy RS, Joyce AP, Roberts S, Buxton TB, Liewehr F. An
in vitro evaluation of the antibacterial efficacy of chlorine dioxide on E. faecalis in bovine incisors. J Endod. 2005. 31:672–675.

19. Kuruvilla JR, Kamath MP. Antimicrobial activity of 2.5% sodium hypochlorite and 0.2% chlorhexidine gluconate separately and combined, as endodontic irrigants. J Endod. 1998. 24:472–476.

20. Ringel AM, Patterson SS, Newton CW, Miller CH, Mulhern JM.
In vivo evaluation of chlorhexidine gluconate solution and sodium hypochlorite solution as root canal irrigants. J Endod. 1982. 8:200–204.

21. Harrison JW, Hand RE. The effect of dilution and organic matter on the anti-bacterial property of 5.25% sodium hypochlorite. J Endod. 1981. 7:128–132.

22. Vahdaty A, Pitt Ford TR, Wilson RF. Efficacy of chlorhexidine in disinfecting dentinal tubules in vitro. Endod Dent Traumatol. 1993. 9:243–248.
23. Dametto FR, Ferraz CC, De Almeida Gomes BP, Zaia AA, Teixeira FB, De Souza-Filho FJ.
In vitro assessment of the immediate and prolonged antimicrobial action of chlorhexidine gel as an endodontic irrigant against Enterococcus faecalis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005. 99:768–772.

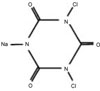
24. Lee WC, Kang BS, Kim CH, Son HH. Evaluation of sodium dichloroisocyanurate as a root canal irrigation solution; Cl- concentration, pH, cytotoxicity and antimicrobial effect
in vitro. J Korean Acad Conserv Dent. 2003. 28:425–430.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download