Abstract
To evaluate the effect of vital tooth bleaching agent and alcohol pretreatment on dentin bonding, flat dentin windows were produced on the buccal side of the crowns of fifty-five extracted, human premolars. A bleaching gel, Opalescence® with 10% of carbamide peroxide (Ultradent Product, USA) was daily applied on the teeth of three experimental groups for six hours for 10 consecutive days, while teeth of a control group were not bleached. After 6 hours of bleaching gel application, the specimens were washed and stored in saline until the next day application. After application of One-step® dentin bonding agent (Bisco, USA), Z-250® resin (3M-ESPE, USA) was bonded to dentin with a mount jig. Shear bond strength was measured with an Instron machine (Type 4202, Instron Corp., USA) after 24 hours. The results were analyzed using one-way ANOVA and Duncan's multiple range test at p < 0.05.
Immediate bonding group showed significantly lower bond strength than un-bleached control group (p < 0.05).
Ethanol-treated group showed significantly higher bond strength compared to immediate bonding group (p < 0.05). However, the bond strength of the ethanol treatment group was lower than that of the un-bleached control group (p < 0.05).
There were no significant difference in shear bond strength between the 2-week delayed bonding group and the ethanol-treated group (p > 0.05) and between delayed bonding group and un-bleached control group (p > 0.05).
In the condition of the present study, it seems that alcohol pretreatment after bleaching procedure can reduce the adverse effect of vital bleaching agent on dentin bonding.
As more emphasis is placed on esthetics, tooth discoloration is becoming a greater concern. Many different agents to bleach discolored teeth have been used. Vital bleaching with 10% carbamide peroxide, also known as nightguard vital bleaching, became a standard technique1,2). Since Haywood and Heymann3) first reported this technique in 1989, vital bleaching combined with esthetic restorative treatment has been an important aspect of esthetic dentistry.
Hydrogen peroxide is an active bleaching ingredient in carbamide peroxide. Hydrogen peroxide releases free radicals which act as oxidants4). These oxidants react with and cleave the bonds to the chromophores (color radicals). The 10% carbamide peroxide solutions have a 3.0% to 3.5% equivalent concentration of hydrogen peroxide, an effective yet safe level for tooth whitening that is more diluted than the potent 35% hydrogen peroxide concentrations4). Nightguard vital bleaching using 10% carbamide peroxide has received much attention as an effective and simple method for the improvement of the appearance of discolored teeth. However, some authors speculate that residual peroxide or oxygen radicals in bleached teeth inhibit the polymerization of resin and adversely affect the bond strength of composite to enamel and dentin5-7).
Among the several methods to minimize the reduction of bond strength after bleaching process8,9), the most common method is to delay bonding procedures for 2 weeks after bleaching10,11). From recent studies12,13), the adverse effect of bleaching on enamel bonding could also be reduced or eliminated by treating the bleached surface with alcohol or acetone-based adhesives. Therefore it would be possible without delay to bond the bleached surface with esthetic restoration materials. However, the effects of these new treatment after tooth bleaching on dentin bond strength have not been thoroughly studied.
The objective of this in vitro study was to evaluate the effect of a vital bleaching agent and alcohol pretreatment on dentin bonding.
Fifty five extracted, caries-free human premolars that had been stored in saline were used in this study. The crowns of teeth were cut at the cemento-enamel junction using a diamond bur under copious water irrigation. The crowns were embedded in auto-polymerizing acrylic resin (Orthodontic Resin, Dentsply/Detray, Konstanz, Germany) molds so that the prepared buccal enamel surfaces were 5 mm above the acrylic resin cylinders. The teeth in molds were placed in tap water to prevent the temperature rise by the exothermic polymerization reaction.
After the resin had set, the buccal enamel was removed using a high-speed diamond bur. A flat dentin window was produced using 220-, 400-, and 600-grit silicon carbide abrasive papers on a water-cooled, model trimming wheel to make uniform bonding condition. The teeth were randomly divided into four groups (Table 1).
Opalescence® (Ultradent Product, Inc., Salt Lake City, UT, USA) with 10% carbamide peroxide gel was used as bleaching material. Teeth of three experimental groups were daily applied with bleaching gel for six hours for 10 consecutive days while teeth of the control group were not bleached. After 6 hours of bleaching gel application, the specimens were washed and stored in saline until the next day application. Saline was used for the storage of the specimens rather than artificial saliva, to prevent the interference of other variables.
Teeth in immediate bonding group and delayed bonding group, no treatment was applied on the bleached dentin surface. Teeth in ethanol-treated group were treated with 70% ethanol. 70% ethanol was applied on the bleached dentin surface for 3 minutes with a cotton pellet of burnishing motion and subsequently washed with saline for 2 minutes.
The flattened and bleached surfaces were etched with 37% phosphoric acid gel for 15 seconds, and dried with air syringe for 20 seconds. One-step® (Bisco, Inc., Schaumburg, Illinois, USA) was used as a bonding agent and applied following the manufacturer's instructions.
A mount jig (Ultradent Product Inc., South Jordan, Utah, USA) with an internal ring of 2.3798 mm in diameter and 2.0 mm height was placed on the dentin surface and stabilized with an alignment tube. Resin composite (Z-250®, 3MESPE, St. Paul, MN, USA) was packed into the mold and light-cured for 40 seconds.
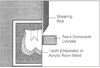
After polymerization, the alignment tube and mold were removed and the specimens were stored at 100% humidity for 24 hours. After storage of 24 hours, the resin-mounted tooth was placed in a jig so that the shear force could be applied perpendicular to the long axis of the resin cylinder (Figure 1). Shear bond strength was measured using Instron testing machine (Type 4202, Instron Corp., Canton, Massachusetts, USA) at a crosshead speed of 1 mm/minute.
The results of shear bond strength tests were statistically analyzed using one-way ANOVA, with differences among the groups determined by Duncan's multiple range test with a confidence level of 95%.
The results of the shear bond strength test for the four test groups are shown in Figure 2.
Immediate bonding group showed significantly lower bond strength than un-bleached control group (p < 0.05).
Ethanol-treated group showed significantly higher bond strength compared to immediate bonding group (p < 0.05). However, the bond strength of the ethanol treatment group was lower than that of the un-bleached control group (p < 0.05).
There were no significant differences in shear bond strength between the 2 week-delayed bonding group and the ethanol-treated group (p > 0.05) and between delayed bonding group and un-bleached control group (p > 0.05).
Since composite resin with bonding agent can be applied to dentin after vital tooth bleaching procedure, the present study was designed to evaluate the effect of a vital bleaching agent and alcohol pretreatment on dentin bonding. In the present study, there was no statistically significant difference in shear bond strength between the 2-week delayed bonding group and the ethanol treatment group. The results suggest that it would be possible to bond the bleached dentin surface with esthetic restoration materials without delay.
In the present study, bleached, immediate bonding group showed significantly lower bond strength than un-bleached control group. There have been studies that bleaching reactions adversely affect the bond strength of adhesive systems and resin composites to enamel and dentin. Although the mechanisms are not totally known, it is hypothesized that as the oxidizing agent hydrogen peroxide diffuses through the dentin and enamel14), the highly pigmented carbon ring compounds are open and converted into chains, which are lighter in color. As this process continues, the bleached tissue continually becomes lightened15) with further decomposition of organic and inorganic matrix. During this process, water and oxygen are released16,17). Peroxides and oxygen may remain on the tooth surface and they could inhibit the polymerization process of the adhesive systems. Some authors have demonstrated that teeth subjected to 10% carbamide peroxide have greater microleakage of composite restorations compared to nonbleached teeth18,19).
Titley et al.20) explained the reduction in adhesiveness of carbamide peroxide bleached enamel on the basis of the interaction between resin and residual peroxide that occurred at the resin composite-enamel interface. Possible effects of this interaction include inhibition of polymerization of the resin by oxygen and an increase in resin porosity created by oxygen gas generation, which could be the result of oxidizing reactions due to the entrapment of peroxide in the subsurface laylayer of the enamel. In a scanning electron microscopic evaluation, nonbleached teeth presented numerous and clearly defined resin tags, in contrast with the teeth treated with carbamide peroxide, where the resin tags were sparse, shorter, poorly defined, and structurally incomplete10). Reduction in bond strength in hydrogen-peroxide treated dentin also could be caused by residual solution in the collagen matrix and dentinal tubules that eventually broke down to oxygen and water21). Liberation of oxygen could either interfere with resin infiltration into etched dentin, or inhibit polymerization of resins7).
There have been proposed several methods to reverse the reduced bond strength after bleaching22). To delay any bonding procedures for 2 weeks, which is the period for natural decomposition of residual peroxide23) after bleaching, is common recommendation10,24). In the present study, the bleached, 2-week delayed resin bonding group was included. There were no statistically significant difference in shear bond strength between 2-week delayed bonding group and un-bleached group. Although this 2-week delay method is most common, when an immediate esthetic restoration of the bleached tooth is required, prolonged waiting may not be feasible.
Removal of the superficial layer has been suggested to induce complete reversal of the reduced enamel bond strength. Cvitko et al.25) suggested that flattening of the enamel surface ranging from 0.5 to 1.0 mm after bleaching increased bond strength to nearly non-bleached control value since residual oxygen is present only in the outer surface of the enamel. It may not be possible to remove the layer of dentin because depth and amount of residual oxygen remaining in teeth depend on tooth bleaching method, enamel thickness, concentration of the bleaching agent, and the bleaching time23).
There is a method to pretreat the bleached surface with alcohol or acetone-based adhesives26). In the present study, there were no statistically significant difference in shear bond strength between the 2-week delayed bonding group and the ethanol-treated group. Kalili et al.26) proposed that inhibition of polymerization of resin could result from bleaching agents that cause oxygen to penetrate and concentrate on the surface of enamel, thus inhibiting the cure of some resin tags. They also suggested that the alcohol pretreatment to bleached enamel may have been able to minimize the inhibitory effects of the bleaching agent. Sung et al.13) also suggested that the use of alcohol-based agents may result in less compromised composite bond strength when restorative work is to be completed immediately after bleaching. From the study of Kum et al.12), ethanol removed residual peroxide from enamel by extracting water.
Additionally, there has been a method to apply catalase to bleached tooth surface prior to bonding. Catalase is an essential enzyme for the proper function of the body defense mechanism. It is an oxygen scavenging enzyme normally present abundantly in mammalian tissues known to be involved in the decomposition of hydrogen peroxide to water and oxygen. From the study of Rostein et al.27), there was a protective effect of catalase against the hydrogen peroxide-induced injury in rat oral mucosa. It has been found that the catalase application to teeth immediately after bleaching eliminated the hydrogen peroxide residues and prevented their radicular penetration28). Even though catalase is an effective adjunct following bleaching to remove the residual peroxide, it is not clinically practical as it has a short shelf life and is sensitive to air. Hence, it needs to be refrigerated for storage12). On the other hand, compared to catalase, ethanol does not need any special storage and is easily available at chair side, and is a clinically useful agent for reducing the adverse effect of bonding by residual peroxide.
From the results of the present study, it seems that pretreatment of bleached dentin surface with ethanol significantly improves the resin-dentin bond strength compared to bleached, immediately resin bonding, which is comparable to the 2-week delayed resin bonding. Therefore, it could be concluded that, under the conditions of this study, alcohol pretreatment after bleaching procedure can reduce the adverse effect of vital bleaching agent on dentin bonding. Further studies are necessary to quantitatively evaluate the residual hydrogen peroxide with and without alcohol pretreatment on dentin.
Figures and Tables
References
1. Wandera A, Feigal RJ, Douglas WH, Pintado MR. Home-use tooth bleaching agents: an in vitro study on quantitative effects on enamel, dentin, and cementum. Quintessence Int. 1994. 25:541–546.
2. Covington JS, Friend GW, Lamoreaux WJ, Perry T. Carbamide peroxide tooth bleaching: effect on enamel conposition and topography. J Dent Res Special Issue. 1990. 530.
3. Haywood VB, Heymann HO. Nightguard vital bleaching. Quintessence Int. 1989. 20(3):173–176.
4. García-Godoy F, Dodge WW, Donohue M, O'Quinn JA. Composite resin bond strength after enamel bleaching. Oper Dent. 1993. 18:144–147.
5. Torneck CD, Titley KC, Smith DC, Adibfar A. The influence of time of hydrogen peroxide exposure on the adhesion of composite resin to bleached bovine enamel. J Endod. 1990. 16:123–128.

6. Titley KC, Torneck CD, Ruse ND, Krmec D. Adhesion of a resin composite to bleached and unbleached human enamel. J Endod. 1993. 19:112–115.

7. Torneck CD, Titley KC, Smith DC, Adibfar A. Adhesion of light-cured composite resin to bleached and unbleached bovine dentin. Endod Dent Traumatol. 1990. 6:97–103.

8. Kaya AD, Turkun M. Reversal of dentin bonding to bleached teeth. Oper Dent. 2003. 28:825–829.
9. Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993. 300:535–543.

10. Titley KC, Torneck CD, Ruse ND. The effect of carbamide-peroxide gel on the shear bond strength of a microfil resin to bovine enamel. J Dent Res. 1992. 71:20–24.

11. Spyrides GM, Perdigao J, Pagani C, Araujo MA, Spyrides SM. Effect of whitening agents on dentin bonding. J Esthet Dent. 2000. 12:264–270.

12. Kum KY, Han YS, Jung IY, Lee SJ, Lee CY, Oh BH. A quantitative study on the degrading effect of the various cavity irrigating agents in the elimination of residual hydrogen peroxide following walking bleaching. J Korean Acad Conserv Dent. 1998. 23(2):656–669.
13. Sung EC, Chan SM, Mito R, Caputo AA. Effect of carbamide peroxide bleaching on the shear bond strength of composite to dental bonding agent enhanced enamel. J Prosthet Dent. 1999. 82:595–599.

14. Gökay O, Mujdeci A, Algin E. Peroxide penetration into the pulp from whitening strips. J Endod. 2004. 30:887–889.

15. Kwon YH, Huo MS, Kim KH, Kim SK, Kim YJ. Effects of hydrogen peroxide on the light reflectance and morphology of bovine enamel. J Oral Rehabil. 2002. 29:473–477.

16. Goldstein GR, Kiremidjian-Schumacher L. Bleaching: is it safe and effective? J Prosthet Dent. 1993. 69:325–328.

17. Leonard RH Jr, Bentley CD, Haywood VB. Salivary pH changes during 10% carbamide peroxide bleaching. Quintessence Int. 1994. 25:547–550.
18. Shinohara MS, Rodrigues JA, Pimenta LA. In vitro microleakage of composite restorations after nonvital bleaching. Quintessence Int. 2001. 32:413–417.
19. Türkün M, Turkun LS. Effect of nonvital bleaching with 10% carbamide peroxide on sealing ability of resin composite restorations. Int Endod J. 2004. 37:52–60.

20. Titley KC, Torneck CD, Smith DC, Chernecky R, Adibfar A. Scanning electron microscopy observations on the penetration and structure of resin tags in bleached and unbleached bovine enamel. J Endod. 1991. 17:72–75.

21. Lai SC, Mak YF, Cheung GS, Osorio R, Toledano M, Carvalho RM, Tay FR, Pashley DH. Reversal of compromised bonding to oxidized etched dentin. J Dent Res. 2001. 80:1919–1924.

22. Lai SC, Tay FR, Cheung GS, Mak YF, Carvalho RM, Wei SH, Toledano M, Osorio R, Pashley DH. Reversal of compromised bonding in bleached enamel. J Dent Res. 2002. 81:477–481.

23. Kum KY, Lim KR, Lee CY, Park KH, Safavi KE, Fouad AF, Spangberg LS. Effects of removing residual peroxide and other oxygen radicals on the shear bond strength and failure modes at resin-tooth interface after tooth bleaching. Am J Dent. 2004. 17:267–270.
24. Kim KK, Park JW. Comparison of shear bond strength of different bonding systems on bleached enamel. J Korean Acad Conserv Dent. 2004. 29:30–35.

25. Cvitko E, Denehy GE, Swift EJ, Pires KA. Bond strength of composite resin to enamel bleached with carbamide peroxide. J Esthet Dent. 1991. 3:100–102.

26. Kalili KT, Caputo AA, Yoshida K. Effect of alcohol pretreatment on composite bond strength to bleached enamel. J Dent Res. 1993. 72:283.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download