Abstract
The aim of this in vitro study was to evaluate the suitability of using chitosan, poly (lactide-co-glycolide) (PLGA), and polymethyl methacrylate (PMMA) to control the release of chlorhexidine digluconate (CHX) from a prototype of controlled release drug device (CRD) for root canal disinfection. Four different prototypes with different formulations were prepared. Group A (n = 12); The device (absorbent paper point) was loaded with CHX as control. Group B (n = 12); same as group A, but the device was coated with chitosan. In Groups C and D, the device was treated in the same way as group A and then coated three times with 5% PMMA (Group C, n = 12), or coated three times with 3% PLGA (Group D, n = 12). The devices were randomly allocated to experimental groups of 12 each.
All CRD prototypes were soaked in 3 mL distilled water. The concentrations of CHX were determined using a UV spectrophotometer. The surface characteristics of each prototype were observed using a scanning electron microscope.
The result showed that release rate of CHX was the greatest in the non-coated group, followed by the chitosan-coated group, the PLGA-coated group, and the PMMA-coated group (P < 0.05). Pores were observed on the surface of the prototypes that were coated with PLGA and PMMA. When the pore size was smaller, the release rate was lower. This data indicate that polymer coating can control the release rate of CHX from the CRD prototypes.
Complete debridement and effective disinfection of the root canal space are considered essential for predictable long-term success of endodontic treatment1). However, instrumentation and irrigation is not always effective in eliminating a therapy-resistant microflora in the root canal system1-3). Calcium hydroxide has proven to be an excellent antimicrobial agent for intracanal dressing in the treatment of infected root canals4,5). However, it is known to be less effective against Enterococcus faeclais, Actinomyces and Candida that are frequently isolated in persistent/infected root canals6). The antimicrobial efficacy of calcium hydroxide to affect microorganisms entrenched in the dentinal tubules is also questionable7). Therefore, alternative medicaments should be explored that would maximize microbial eradication when used as intracanal dressings.
Chlorhexidine is effective against a wide variety of Gram-positive and Gram-negative organisms, as well as fungi. Recent studies have shown that the antimicrobial effect of chlorhexidine digluconate (CHX) was equal to that of the conventional irrigants and medicaments8-11). In addition, it is retained by the dentinal hard tissues and thus has a substantive antimicrobial action12-14). It was also suggested as an effective irrigant to prevent root canal reinfection due to coronal leakage15). However, in order to achieve long-term substantive antimicrobial effect, the infected root dentin must be exposed to CHX for a longer time than that afforded by irrigation16,17).
A number of studies have shown that a controlled release drug (CRD) device with water-permeable polymer could effectively sustain the release of CHX from the CRD16,17). However, because of a strong, positive charge of chlorhexidine and its high binding affinity, the development of suitable drug carriers for sustained release of CHX still remains a challenge.
Chitosan, poly (lactide-co-glycolide) (PLGA), and polymethyl methacrylate (PMMA), are well known polymers as controlled drug release carrier. Miyazaki et al. observed the sustaining effect of chitosan on the release of water insoluble indomethacin from granules18). A sustained plateau level of indometacin was obtained for drug/chitosan granules (1 : 2 mixtures) versus a sharp peak for conventional commercial capsules in a rabbit model. PLGA is one of the best-known biodegradable polymers. It is hydrolyzed without enzymes and metabolized by the body19). Moreover, the degradation rate of PLGA can be regulated by changing its molecular weight, chemical composition, and crystal form20). PMMA has been used as denture base materials, and one recent study suggested that it could be used as a controlled drug release carrier for antibiotics, for the prevention and treatment of osteomyelitis21). Therefore, all three polymers may be promising controlled drug release carriers.
The aim of this in vitro study was to compare the sustaining effect of chitosan, PLGA, and PMMA on the release of CHX from a prototype of CRD device for root canal disinfection.
CHX solution (20% wt / wt, Sigma, St. Louis, MO, USA) was diluted serially in 1:1 ratios, and the UV absorbance was measured for each dilution using a UV spectrophotometer (Shimadzu, Tokyo, Japan). The standard curve of CHX concentration versus UV absorbance was used to determine CHX concentration in the experiments.
Absorbent paper points (Sure-Endo™, #80, Chungju, Korea) were used as core material. Four different prototypes with different formulations were prepared: group A; absorbent paper points were loaded with CHX. The paper points were immersed in 40% concentrated CHX solution obtained by drying process for 30 minutes and then dried. The 40% concentrated CHX solution was obtained by evaporating water of 20% CHX solution in an oven at 50oC until target weight was reached. Group B; after loading with CHX as in group A, the paper points were coated with an acidic aqueous 3% solution of chitosan (Texan MedTech, Kwangju, Korea) and dried. Groups C and D were treated as Group B except that the paper points were coated three times with 5% PMMA (Group C, Aldrich®, Milwaukee, WI, USA) in methylene chloride, or three times with 3% PLGA (Group D, Sigma®, St. Louis, MO, USA) in methylene chloride, respectively. For Group C and D, the CHX-loaded paper points were dip-coated with polymer solutions and dried, and this process was repeated twice. All loaded absorbent paper points were individually weighed before being coated. The ones with the range of 0.033 ± 8.43 × 10-5 g were selected, and they were randomly allocated to experimental groups of 12 each.
Each prototype was immersed in 3 ml of distilled water. 10µl of this solution was then sampled at predetermined times (i.e., at 3, 6, 10, 20, 30, 40 and 50 min and at 1, 2, 3, 4, 5 and 6h, and at 7days). UV absorbance was measured using a UV spectrophotometer (Shimadzu, Tokyo, Japan) to determine the concentration of released CHX from the CRD prototype.
The average weight of CHX loaded in the paper points was 0.016g/point. If all the CHX loaded in the paper point was released into 3mL of distilled water, the concentration would have been about 0.53%. From Figure 1, we calculated that the UV absorbance of 0.53% CHX was about 1.1, which thus represented the maximum UV value.
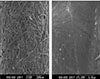
Scanning electron micrographs showed the different surface characteristics of the CRD prototypes. In the non-coated group, the fiber structure of the absorbent paper point was unaffected and no surface pores were observed (Figure 4). In the polymer-coated groups, coated fiber structure was observed in all prototypes. However, the surface pores were only observed in the PMMA- and PLGA-coated groups, and the pore sizes differed between the two groups. The pore size of the PLGA-coated group is about 2 µm and larger than that of the PMMA-coated group which is <1 µm in size (Figures 5 and 6). The chitosan covered absorbent paper points did not show any pores (Figure 7).
Huang et al.22) developed the cylindrical, needle-shaped CRD prototypes with different formulations and demonstrated that the releasing rate of CRD with non-coated formulation was very fast. In contrast, the release rate of CRD with coated formulations was far more controlled.
In this study, similar results were obtained. In the non-coated group, the drug release was very fast, and all loaded CHX was released within 2h. In contrast, CHX release from the polymer-coated groups was more controlled. Chitosan was more sensitive to water and easily swollen with water and ruptured. This resulted in faster release of CHX from the chitosan-coated CRD device compared to the PLGA- and PMMA-coated groups. The CHX loaded in the paper point was released through the surface pores on the coated polymer layer. The pore size of PLGA-coated group was larger than that of PMMA-coated group and the release rate of CHX from the latter group was lower than that of the former group. Thus, the surface pore size was very important for the release rate of CHX and various release rates of CHX from CRD devices can be achieved by controlling the pore size of the coated polymer. The ideal CRD device should have the following characteristics. It should not degrade inside the root canal and it should be easily inserted into and removed from the root canal. In addition, the drug should be released continuously for a controlled time period. Heling et al16,17) developed a CRD device containing a biodegradable polymer and demonstrated that it was more effective than calcium hydroxide at disinfecting dentinal tubules. However, if used for root canal disinfection, it may not be completely degraded at the time for root filling. Any remaining fragments in the root canal may interfere with the permanent filling, and thus result in leakage.
Due to this concern, insoluble polymers were used for coating. Chitosan is insoluble at an alkaline or neutral pH23). PMMA, which has been used for denture base, is also an insoluble and nondegradable material. PLGA is a biodegradable polymer, but the degradation rate of PLGA can be controlled using the lactide to glycolide mole ratio19). Therefore, all the materials used in the present study are suitable as coatings for drug carrier for root canal disinfection. The use of absorbent paper point as core material can easily be inserted into root canals and they can be easily removed from the root canal after use.
Based on the above results, we conclude that the polymer coating can effectively control the release rate of CHX from the CRD prototypes. Further studies are needed to evaluate the antimicrobial effects and the cytotoxicity of these prototypes of CRD device.
Figures and Tables
 | Figure 1Standard graph of CHX concentration and UV absorbance (Y = 1.99X; X axis: concentration of CHX, Y axis: UV absorbance). |
 | Figure 2Short-term release rate of CHX after immersion of controlled release drug device in 3 ml of distilled water. |
 | Figure 3Long-term release rate of CHX after immersion of controlled release drug device in 3 ml of distilled water. |
 | Figure 4SEM images of the non-coated paper point which was loaded with CHX; (a) 100× (b) 5000×; the fiber structure of the paper point was observed without pores. |
 | Figure 5SEM images of a prototype of controlled release drug device coated with 5% PMMA three times; (a) 100×, (b) 5000×.; surface pores were observed, and all were within 1 µm. |
References
1. Sjogren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997. 30:297–306.

2. Sundqvist G, Figdor D, Persson S, Sjogren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998. 85:86–93.

3. Friedman S, Komorowski R, Maillet W, Klimaite R, Nguyen HQ, Torneck CD. In vivo resistance of coronally induced bacterial ingress by an experimental glass ionomer cement root canal sealer. J Endod. 2000. 26:1–5.

4. Bystrom A, Claesson R, Sundqvist G. The antimicrobial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985. 1:170–175.

5. Cvek M, Hollender L, Nord CE. Treatment of non-vital permanent incisors with calcium hydroxide. Odontol Revy. 1976. 27:93–108.
6. Orstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings by experimentally infected dentinal tubules. Endod Dent Traumatol. 1990. 6:142–149.

7. Haapasalo M, Ørstavik D. In-vitro infection and disinfection of dentinal tubules. J Dent Res. 1987. 66:1375–1379.
8. Gomes BPFA, Souza SFC, Ferraz CCR, Teizeira FB, Zaia AA, Valdrigh L, Souza-Filho FJ. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int Endod J. 2003. 36:267–275.

9. Basrani B, Tajderhane L, Santos M, Pascon E, Grad H, Lawrence HP, Friedman S. Efficacy of chlorhexidine- and hydroxide-containing medicaments against Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003. 96:618–624.

10. Vianna ME, Gomes BP, Berber VB, Zaia AA, Ferraz CC, de Souza-Filho FJ. In vitro evaluation of the antimicrobial activity of chlorhexidine and sodium hypochlorite. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004. 97:79–84.

11. Jeansonne MJ, White RR. A comparison of 2% chlorhexidine gluconate and and 5.25% sodium hypochlorite as antimicrobial endodontic irrigants. J Endod. 1994. 20:276–278.

12. White RR, Hay GL, Janer LR. Residual antimicrobial activity after canal irrigation with chlorhexidine. J Endod. 1997. 23:229–231.

13. Basrani B, Santos JM, Tjaderhane L, Grad H, Gorduysus O, Huang J, Lawrence HP, Friedman S. Substantive antimicrobial activity in chlorhexidine-treated human root dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002. 94:240–245.

14. Komorowski R, Grad H, Wu Y, Friedman S. Antimicrobial substantivity of chlorhexedine-treated bovine root dentin. J Endod. 2000. 26:315–317.
15. Jung S, Safavi K, Spangberg L. The effectiveness of Chlorhexidine in the prevention of root canal reinfection [abstract]. J Endod. 1999. 25:288.

16. Heling I, Sommer M, Steinberg D, Friedman M, Sela MN. Microbiological evaluation of the efficacy of chlorhexidine in a sustained-release device for dentine sterilization. Int Endod J. 1992. 25:15–19.

17. Heling I, Steinberg D, Kenig S, Gavrilovich I, Sela MN, Friedman M. Efficacy of a sustained-release device containing chlorhexidine and Ca (OH)2 in preventing secondary infection of dentinal tubules. Int Endod J. 1992. 25:20–24.

18. Miyazaki S, Yamaguchi H, Yokouchi C, Takada M, Hou WM. Sustained-release and intragastric-floating granules of indomethacin using chitosan in rabbits. Chem Pharm Bull. 1988. 36:4033–4038.

19. Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microsphers. Adv Drug Deliv Rev. 1997. 28:5–24.
20. Cam D, Hyon SH, Ikada Y. Degradation of high molecular weight poly (L-lactide) in alkaline medium. Biomaterials. 1995. 16:833–843.

21. Bayston R, Milner RDG. The sustained release of antimicrobial drugs from bone cement. J Bone Joint Surg Br. 1982. 64:460–464.
22. Huang J, Wong HL, Zhou Y, Wu XY, Grad H, Komorowski R. In vitro studies and modeling of a controlled-release device for root canal therapy. J Control Release. 2000. 67:293–307.

23. Singla AK, Chawla M. Chitosan: some pharmaceutical and biological aspects-an update. J Pharm Pharmacol. 2001. 53:1047–1067.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download