1. Council on Dental Materials, Instruments, and Equipment. Visible light-cured and activating units. J Am Dent Assoc. 110(1):100–102. 1985.
2. Blankenau RJ, Kelsey WP, Powell GL, Shearer GO, Barkmeier WW, Cavel WT. Degree of composite resin polymerization with visible light and argon laser. Am J Dent. 4(1):40–42. 1991.
3. Ferracane JL, Mitchem JC, Condon JR, Todd R. Wear and marginal breakdown of composites with various degrees of cure. J Dent Res. 76(8):1508–1516. 1997.

4. Rueggeberg FA, Twiggs SW, Caughman WF, Khajotia S. Life-time intensity profiles of 11 light-curing units. J Dent Res. 75:380 Abstr.. (No. 2897):1996.
5. Friedman J. Variability of lamp characteristics in dental curing lights. J Esthet Dent. 1(6):189–190. 1989.

6. Martin FE. A survey of the efficiency of visible light curing units. J Dent. 26(3):239–243. 1998.

7. Barghi N, Berry T, Hatton C. Evaluating intensity output of curing lights in private dental offices. J Am Dent Assoc. 125(7):992–996. 1994.

8. Miyazaki M, Hattori T, Ichiishi Y, Kondo M, Onose H, Moore BK. Evaluation of curing units used in private dental offices. Oper Dent. 23(2):50–54. 1998.
9. Mills RW. Blue light emitting diodes: Another method of light curing? Br Dent J. 178:169. 1995.
10. Nakamura S, Mukai T, Senoh M. Candela-class high brightness InGaN/AlGaN double heterostructure bluelight-emitting diodes. Appl Phys Lett. 64:1687–1689. 1994.
11. Duke ES. Light-emitting diodes in composite resin photopolymerization. Compend Contin Educ Dent. 22(9):722–725. 2001.
12. Yap AU, Soh MS. Thermal emission by different light-curing units. Oper Dent. 28(3):260–266. 2003.
13. Fujibayashi K, Ishimaru K, Takahashi N, Kohno A. Newly developed curing unit using blue light-emitting diodes. Dent Jap. 34:49–53. 1998.
14. Mills RW, Jandt KD, Ashworth SH. Dental composite depth of cure with halogen and blue light emitting diode (LED) technology. Br Dent J. 186(8):388–391. 1999.
15. Stahl F, Ashworth SH, Jandt KD, Mills RW. Light-emitting diode (LED) polymerization of dental composites: flexural properties and polymerization potential. Biomaterials. 21(13):1379–1385. 2000.
16. Mills RW, Uhl A, Jandt KD. Optical power outputs, spectral and dental composite depths of cure, obtained with blue light emitting diode (LED) and halogen light curing units (LCUs). Br Dent J. 193(8):459–463. 2002.
17. Hansen EK, Asumussen E. Reliability of three dental radiometers. Scand J Dent Res. 101(2):115–119. 1993.
18. Leonard DL, Charlton DG, Hilton TJ. Effect of curing-tip diameter on the accuracy of dental radiometers. Oper Dent. 24(1):31–37. 1999.
19. Cook WD. Spectral distribution of dental photopolymerization sources. J Dent Res. 61:1436–1438. 1982.
20. McCabe JF, Carnick TE. Output from visible-light activation units and depth of cure of light-activated composites. J Dent Res. 68(11):1534–1539. 1989.

21. Nomoto R. Effect of light wavelength on polymerization of light-cured resins. Dent Mater J. 16(1):60–73. 1997.

22. Dickens SH, Milos MF. Relationship of dentin shear bond strengths to different laboratory test designs. Am J Dent. 15(3):185–192. 2002.
23. Versluis A, Tantbirojn D, Douglas WH. Why do shear bond tests pull out dentin? J Dent Res. 76(6):1298–1307. 1997.

24. Stansbury JW. Curing dental resins and composites by photopolymerization. J Esthet Dent. 12:300–308. 2000.

25. Hayakawa T, Kikutake K, Nemoto K. Effectiveness of the addition of water-soluble photoinitiator into the self-etching primers on the adhesion of a resin composite to polished dentin and enamel. Dent Mater J. 18(3):324–333. 1999.

26. Burgess JO, DeGoes M, Walker R, Ripps AH. An evaluation of four light-curing units comparing soft and hard curing. Pract Periodontics Aesthet Dent. 11(1):125–132. 1999.
27. Koliniotou-Kubia E, Jacobsen PH. The effect of irradi-antion time on the physicalperoperties of light-cured resins. Clin Mater. 6(1):21–28. 1990.
28. Takahashi A, Sato Y, Uno S, Pereira PN, Sano H. Effects of mechanical properties of adhesive resins on bond strength to dentin. Dent Mater. 18(3):263–268. 2002.

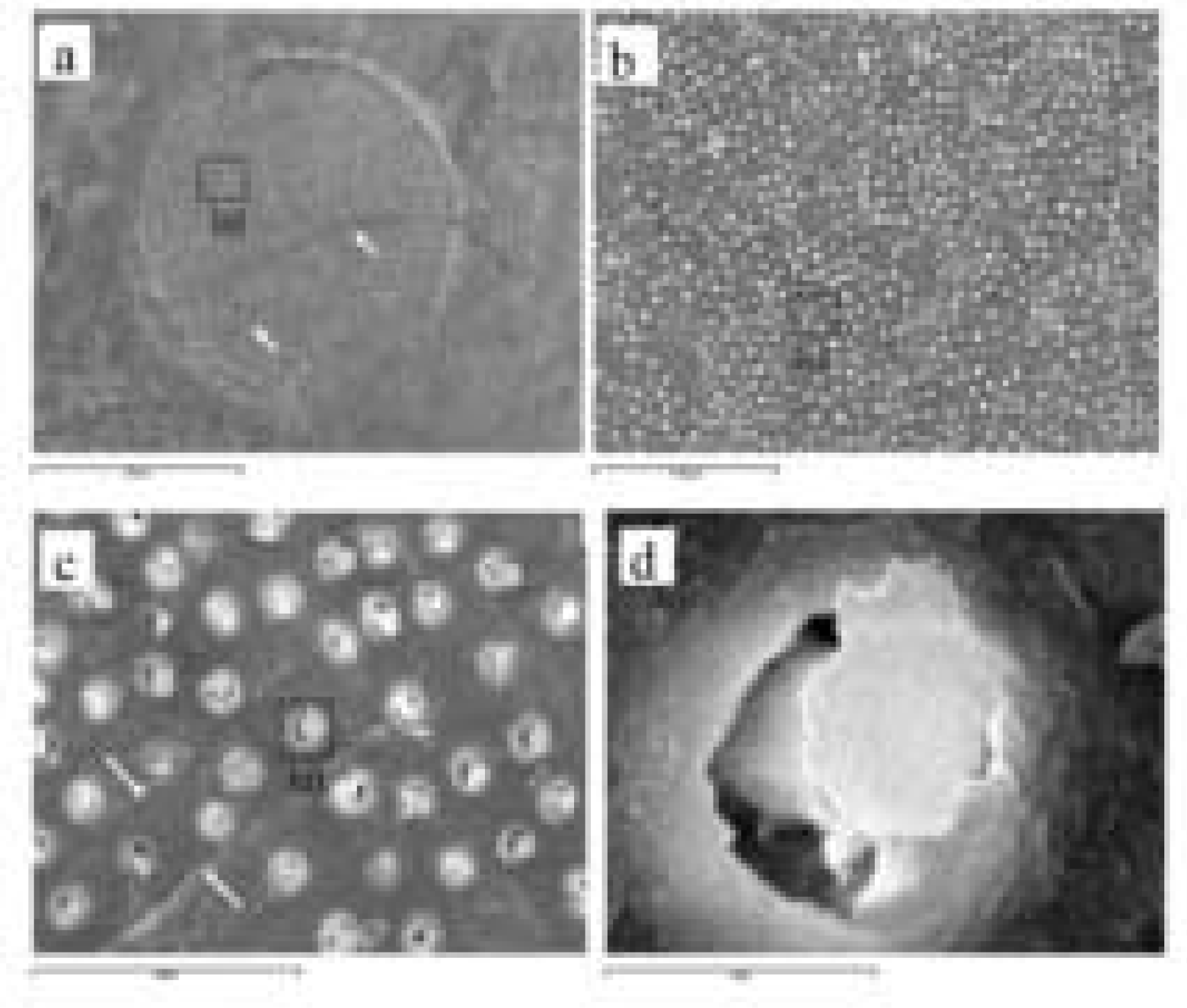

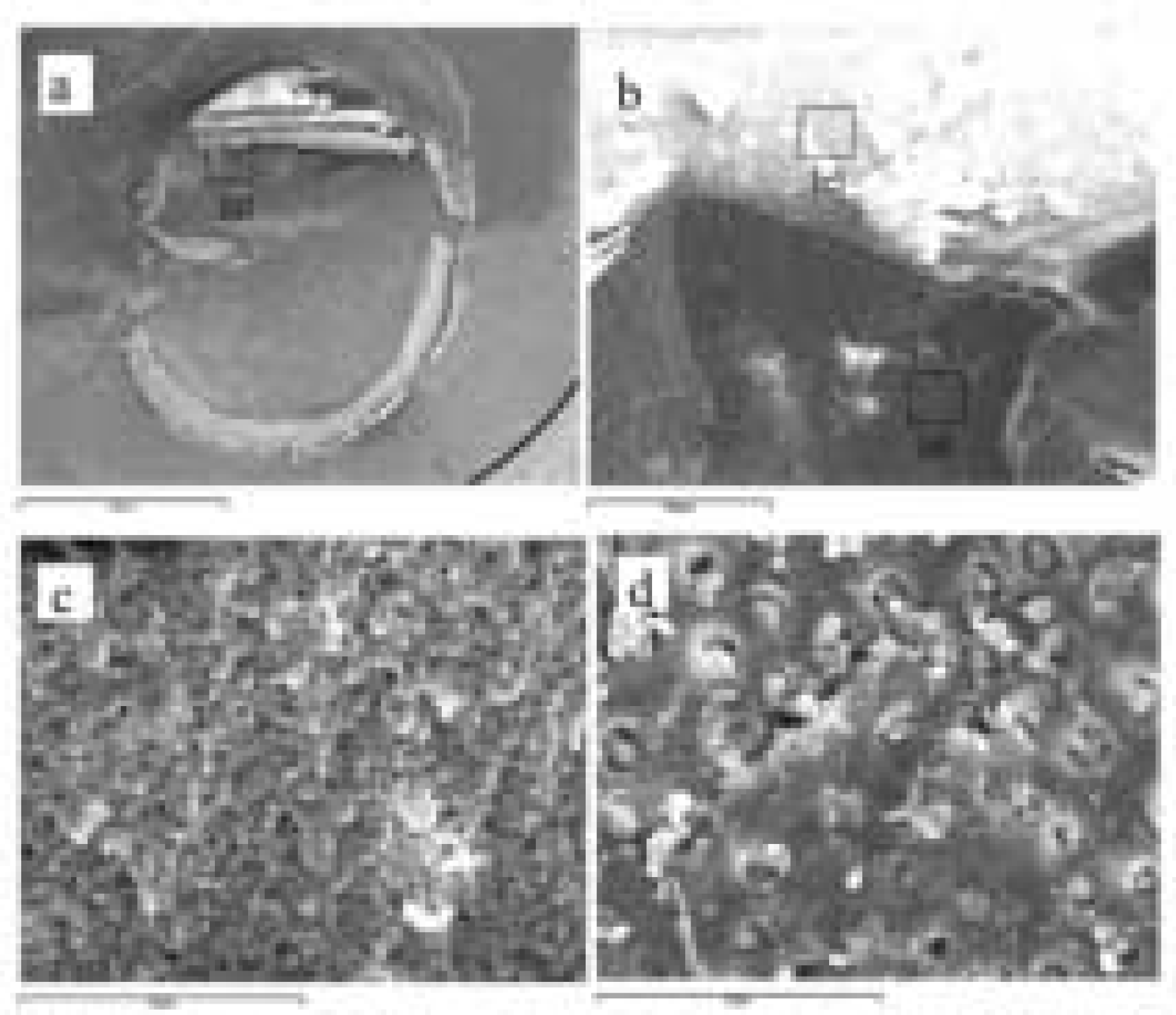




 PDF
PDF ePub
ePub Citation
Citation Print
Print


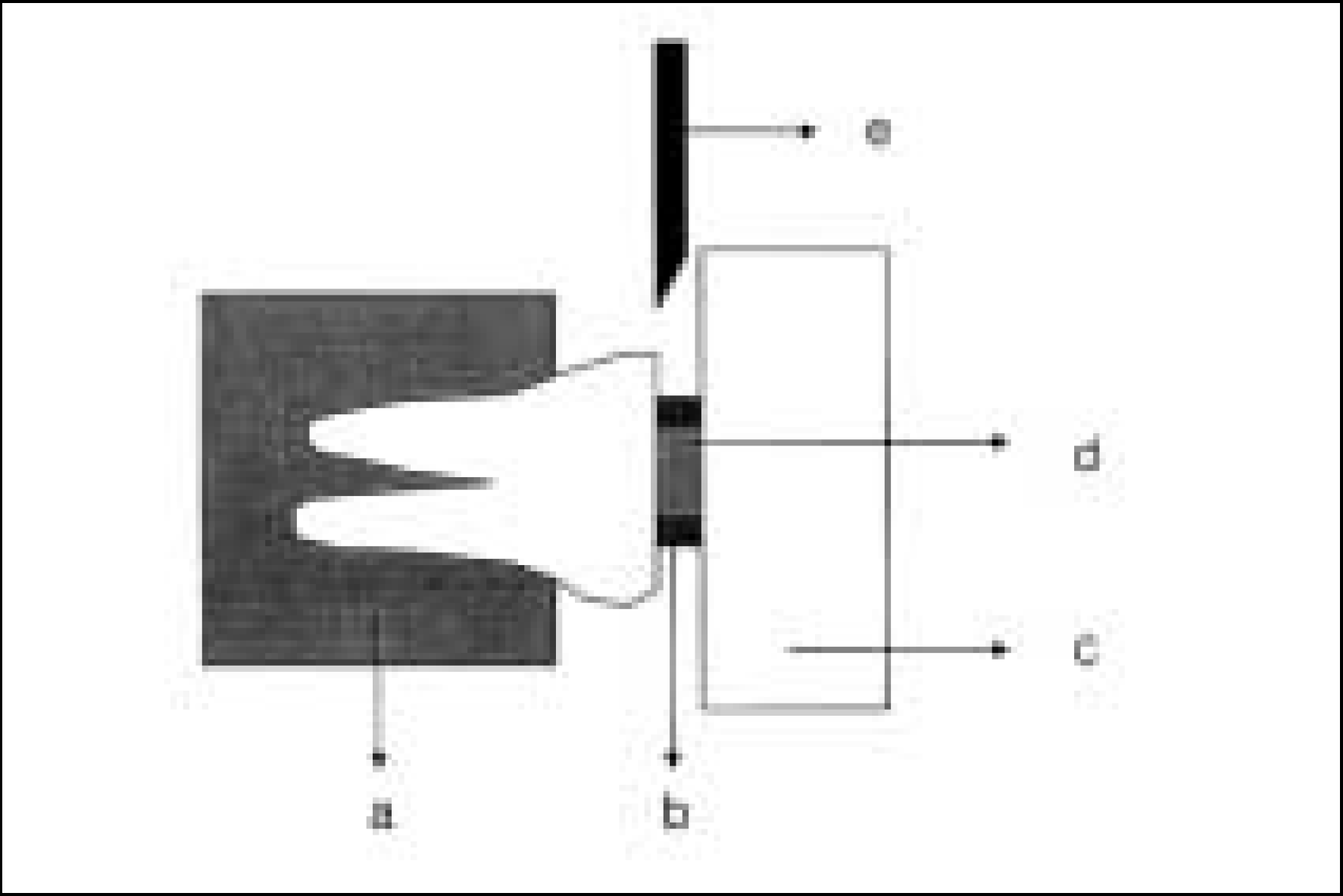
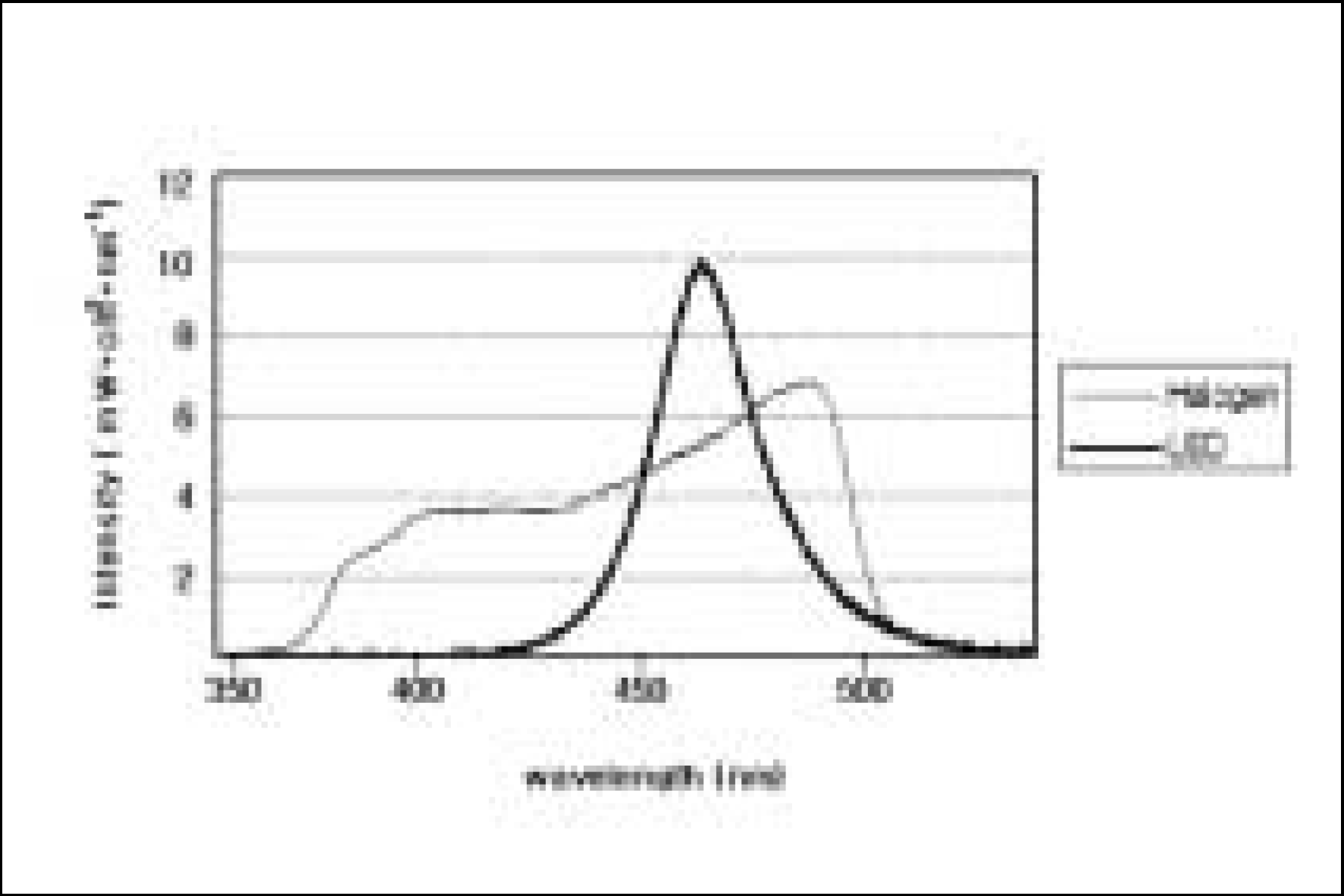
 XML Download
XML Download