Since 1877, the first tooth bleaching was reported by Chapple, many different agents have been used and one of them, nightguard vital bleaching has become an effective and predictable method to lighten discolored teeth. Both 30% hydrogen peroxide-based systems and 10 to 15% carbamide peroxide-based systems have been used successfully in lightening teeth. However, carbamide peroxide has been reported to be less acidic because of the presence of ammonia and carbon dioxide, both of which are by-products of the urea breakdown cycle1). For these reasons, carbamide peroxide bleaching has been suggested as a safer alternative to the harsher hydrogen peroxide-based system2).
Although prerestorative carbamide peroxide bleaching is gaining popularity, its effect on the bond strength to composite has been inconclusive. Carbamide peroxide, major component of current bleaching system, has been implicated in adversely affecting the bond strength of composite to enamel and dentin. There have been reports regarding the interaction between bleaching agents and bond strength of composite materials to enamel; some authors have reported a severe decrease in the average shear bond strength of composite to bleached enamel compared with unbleached enamel3,4). Among the strategies of recovery of the reduced bond strength after bleaching process, delayed bonding method have suggested5,6).
Today, growing efforts are made to simplify and shorten the bonding procedure, by combining the function of conditioner, primer and adhesive. The use of strongly acidic primers introduced the concept of "self-etching" primers not only to dentin but also to enamel, which eliminates the necessity of a separate conditioning step7). The self-etching primer provide comparable bond strengths without the time consuming process of applying and rinsing the etchant8). Fewer steps in the bonding process mean fewer operator errors. Because the monomers that cause the etching are also responsible for bonding, the depth of penetration of the monomers to be polymerized is exactly the same as the depth of demineralization, resulting in a complete hybrid layer. Perdigao et al.9) investigated the effect of calcium on enamel and dentin bond strength. They suggested that calcium removal appears to be less detrimental for self-etching primers compared to total-etch adhesives. Self-etching primer showed different etching pattern and the length of resin tag on enamel compared to those etched with phosphoric acid.
Those differences of bonding mechanism might lead to different bond strength on bleached enamel and clinical investigations are necessary to evaluate the potential of recently developed all-in-one self-etching products which combine the functions of conditioner, primer, and adhesive.
The objective of this study is to compare (1) the effect of type of bonding agents; conventional and self-etching adhesive systems and (2) immediate and delayed bonding of hybrid composite resin on the 10% carbamide peroxide bleached enamel.
Sixty eight extracted, noncarious human molars stored at 4℃ in isotonic saline were used in this study. The last 24 hours before beginning the experiment, they were kept in distilled water. The crowns of 68 teeth were cut at the cementoenamel junction (CEJ) using a low speed diamond saw under copious water cooling then the crowns were cut in half in mesio-distal direction. Each section was embedded in auto-polymerizing acrylic resin with enamel surface facing up (Orthodontic Resin, Dentsply/Detray, Konstanz, Germany) so that the prepared enamel surfaces were 2 mm above the acrylic resin cylinders, and placed in tap water to reduce the temperature rise from the exothermic polymerization reaction.
After the resin had completely polymerized, the enamel surfaces were grounded parallel to the long axis of the tooth on a water-cooled, model trimming wheel and #800 abrasive paper to create flat enamel surface and to make uniform bonding condition.
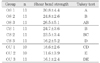
The teeth were randomly divided into nine groups of 11 specimens each and treated in the manner Table 1.
Bleaching material, Opalescence® (Ultradent Product, Inc, Salt Lake City, UT 84095, USA) with 10% of carbamide peroxide gel was used. Control groups were not bleached and stored in saline for 10 days. Bleached groups were exposed to daily application of bleaching gel for six hours during 10 consecutive days. After 6 hours of bleaching gel application, the samples were washed and stored in saline, until the next application. After the bleaching procedure was done, the surface was cleaned with pumice and prepared to bonding composite.
Bonding materials used were One-step® (Bisco., Inc., Schaumburg, Illinois, USA), Clearfil SE Bond® (Kuraray Co., Ltd., Osaka, Japan), and One-up Bond F® (Tokuyama Co., Ltd., Shibutaku, Tokyo, Japan) (Table 2). All bonding materials were applied following the manufacturer's instructions.
Hybrid composite (Clearfil AP-X, Kuraray Co., Ltd., Osaka, Japan) was packed into the Ultradent mount jig mold (Ultradent Product Inc., South Jordan, Utah, USA) and light-cured for 40 seconds. After polymerization, the alignment tube and mold were removed and the specimens were placed in 37℃ distilled water for 24 hours.
Control and immediate bonding groups were tested in shear mode using a chisel-shaped rod in an Instron testing machine (Type 4202, Instron Corp., Canton, Massachusettes, USA) at a crosshead speed of 1 mm/minute. After two-week storage, the groups of delayed bonding specimens were bonded and tested in the same mode.
The data for each group were subjected to oneway ANOVA followed by Tukey's test at p<0.05 to make comparisons among the groups.
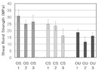
In One-step® and One-up Bond F® groups, the immediate bonding group showed lower bond strength than control and delayed groups.
In Clearfil SE Bond® group, there were no statistical difference of shear bond strength in immediate bonding group compared to control group, but the bond strength of delayed group was lower than the others.
Among bonding agents, One-up Bond F® showed significantly lower bond strength than One-step® and Clearfil SE Bond®.
Although prerestorative carbamide peroxide bleaching is gaining popularity as a more conservative and economical method of improving the esthetics, its detrimental effect on the bond strength to composite has been suggested. Some authors have reported a severe decrease in the average bond strength of composite to bleached enamel compared with unbleached enamel2-6,10).
The exact mechanism of the decreased bond strength after bleaching is not certain, but there were some speculated reasons. One of the reason is that changes in enamel structures resulting from the loss of mineral content, or increased porosity as manifested by an "over-etched" appearance with loss of prismatic form10).
However, Dishman et al.11) argued that the quality of composite bond is compromised through a decreased number of resin tags present, suggesting that there may be some kind of polymerization inhibition taking place. Kalili et al.12) proposed that such inhibition could result from bleaching agents that cause oxygen to penetrate and concentrate on the surface of enamel, thus inhibiting the cure of some resin tags.z
Perdigao et al13) reported that vital bleaching with a commercial 10% carbamide peroxide gel did not change the oxygen concentration in the surface of bleached enamel. And they found that carbamide peroxide bleached enamel showed relatively lower concentration of Ca and P.
To recover the bond strength, some authors have advised of delays in bonding one or more weeks after bleaching because the reduction of composite bond strength to freshly bleached enamel has been transient3,14,15). Similarly, removal of the superficial layer, pretreatment of bleached enamel with alcohol, and use of adhesives containing organic solvents have been also suggested to result in complete reversal of the reduced enamel bonds16-18).
Recently used bonding agents can be divided in two groups by their surface conditioning method. One is conventional total etching system and the other is self-etching system.
Although self-etching primers had lower acidity than conventional etchants, they showed comparable shear bond strengths to conventional types19-21). They explain the reason that the length of resin tags has been shown to contribute little to the bond strength of resin to enamel and bonding is mainly attributable to the ability of the resin to penetrate between the enamel crystallites and rods. If the resin monomers adequately penetrate into the superficially etched surface, that is enough to obtain proper bond strength20).
In control groups, following the results of this study, the conventional acid etching system showed higher bond strength than the self-etching systems and the One-up Bond F®, all-in-one type bonding system, was the lowest.
In the immediate bond strength of OS 2 and CS 2 was comparable but OU 2 group was much lower than the other groups. This can be explained that the lower acidity (higher pH) of self-etching primer. In total etching system, the depletion of calcium from enamel surface could reduce bond strength22,23), but in self-etching system calcium removal from the tooth surface was not detrimental.
The latter system may less influence on the hardness of already weakened enamel structure. In case of using self-etching primer after bleaching, there may be no need to delay bonding steps.
In the delayed bonding groups, the bond strength of OS 3 and OU 3 were recovered to the control group level, but CS 3 was different. We have not known the reason of these results, and more study may be need to reveal those reasons.
In case of One-up Bond F®, there were significantly lower shear bond strength in all test groups than two groups. So it may be used cautiously.
The objective of this study is to compare (1) the effect of type of bonding agents; conventional and self-etching adhesive systems and (2) immediate and delayed bonding of hybrid composite resin on the 10% carbamide peroxide bleached enamel.
68 extracted, noncarious human molars were used in this study. With enamel surface facing up, sectioned teeth were embedded in auto-polymerizing acrylic resin. The teeth were randomly divided into nine groups of 11 specimen each. Bleaching material, Opalescence® with 10% of carbamide peroxide gel was used. Control groups were not bleached and after 10-day storage, those were tested. Experimental groups were exposed to one daily application of bleaching gel for six hours during 10 consecutive days. Used bonding materials were One-step®, Clearfil SE Bond®, One-up Bond F®. All bonding materials were applied per manufacturer's instructions. The immediate bonding groups were bonded the composite right after the final bleaching procedure and delayed groups were immersed in saline and bonded composite. 24 hours after bonding shear bond strength were measured by Instron testing machine at a crosshead speed of 1 mm/minute.
The data for each group were subjected to Oneway ANOVA followed by Tukey's test at p<0.05 to make comparisons among the groups
The results are:
In One-step® groups, the immediate bonding group showed lower bond strength than control and delayed groups.
In Clearfil SE Bond® groups, there were no statistical difference of shear bond strength between the control and immediate groups, but delayed group showed lower bond strength.
In One-up Bond F® groups, the immediate bonding group showed lower bond strength than control and delayed groups. Among bonding agents, One-up Bond F showed significantly lower bond strength than One-step and Clearfil SE Bond.
References
1. Haywood VB, Houck VM, Haymann HO. Nightguard vital bleaching: effects of various solution on enamel surface texture and color. Quintessence Int. 1991. 21:801–804.
2. Stokes AN, Hood JA, Dhariwal D, Patel K. Effect of peroxide bleaches on resin-enamel bonds. Quintessence Int. 1992. 23:769–771.
3. Titley KC, Torneck CD, Ruse ND, Krmec D. Adhesion of a resin composite to bleached and unbleached human enamel. J Endod. 1993. 19:112–115.

4. García-Godoy F, Dodge WW, Donohue M. Composite resin bond strength after enamel bleaching. Oper Dent. 1993. 18:144–147.
5. Torneck CD, Titley KC, Smith DC, Adibfar A. Effect of water leaching on the adhesion of composite resin to bleached and unbleached bovine enamel. J Endod. 1991. 17:156–160.

6. Spyrides GM, Perdigao J, Pagani C, Araujo MA, Spyrides SM. Effect of whitening agents on dentin bonding. J Esthet Dent. 2000. 12:264–270.

7. Pashley D, Sano H. Adhesion testing of dentin bonding agents. Dent Mater. 1995. 11:117–125.
8. Miller RA. Laboratory and clinical evaluation of a self-etching primer. J Clin Orthod. 2001. 35:42–45.
9. Perdigao J, Eriksson S, Rosa BT, Lopes M, Gomes G. Effect of calcium removal on dentin bond strengths. Quintessence Int. 2001. 32:142–146.
10. Ben-Amar A, Liberman R, Gorfil C, Bernstein Y. Effect of mouthguard bleaching on enamel surface. Am J Dent. 1995. 8:29–32.
11. Dishman MV, Covoy DA, Baughan LW. The effects of peroxide bleaching on composite to enamel bond strength. Dent Mater. 1994. 10:33–36.

12. Kalili T, Caputo AA, Mito R, Sperbeck G, Matyas J. Adhesion of a resin composite to bleached and unbleached human enamel. Pract Periodontics Aesthet Dent. 1991. 3:22–24.
13. Perdigao J, Francci C, Swift EJ Jr. Ultra-morphological study of the interaction of dental adhesives with carbamide peroxide-bleached enamel. Am J Dent. 1998. 11:291–301.
14. McGuckin RS, Thurmond BA, Osovitz S. Enamel shear bond strengths after vital bleaching. Am J Dent. 1992. 5:216–222.
15. Miles PG, Pontier JP, Bahiraei D. The effect of carbamide peroxide bleach on the tensile bond strength of ceramic brackets: An in vitro study. Am J Orthod Dentofacial Orthop. 1994. 106:371–375.

16. Cvitko E, Denehy GE, Swift EJ. Bond strength of composite resin to enamel bleached with carbamide peroxide. J Esthet Dent. 1991. 3:100–102.

17. Barghi N, Godwin JM. Reducing the adverse effect of bleaching on composite-enamel bond. J Esthet Dent. 1994. 6:157–161.

18. Sung EC, Chan SM, Mito R, Caputo AA. Effect of carbamide peroxide bleaching on the shear bond strength of composite to dental bonding agent enhanced enamel. J Prosthet Dent. 1999. 82:595–599.

19. Torii Y, Itou K, Hikasa R, Iwata S, Nishitani Y. Enamel tensile bond strength and morphology of resin-enamel interface created by acid etching system with or without moisture and self-etching priming system. J Oral Rehabil. 2002. 29:528–533.

20. Shimada Y, Senawongse P, Harnirattisai C, Burrow MF, Nakaoki Y, Tagami J. Bond strength of two adhesive systems to primary and permanent enamel. Oper Dent. 2002. 27:403–409.
21. Hayakawa T, Nemoto K. Efficacy of self-etching primers in the adhesion of 4-META/MMA-TBB resin cement to enamel. J Adhes Dent. 2002. 4:105–113.
22. Panighi M, G'Sell C. Influence of calcium concentration on the dentin wettability by an adhesives. J Biomed Mater Res. 1992. 26:1081–1089.

23. Swift EJ, Hammel SA, Perdigao J, Wefel JS. Prevention of root surface caries using a dentin adhesive. J Am Dent Assoc. 1994. 125:571–576.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download