Abstract
The purpose of this study was to investigate the influence of NK1 receptor antagonists on the pulpal blood flow (PBF) when applied iontophoretically through the dentinal cavity of the teeth in order to understand whether iontophoretically applied NK1 receptor antagonists can control the pulpal inflammation.
Eleven cats were anesthetized with alpha-chloralose and urethane, and substance P (SP) was administered to the dental pulp through the catheterized lingual artery in doses that caused PBF change without the influence of systemic blood pressure. NK1 receptor antagonists were applied iontophoretically to the prepared dentinal cavity of ipsilateral canine teeth of the drug administration, and PBF was monitored. Data were analyzed statistically with paired t-test.
PBF increase after iontophoretic application of the NK1 receptor antagonists followed by the intra-arterial administration of SP was significantly less than PBF increase after iontophoretic application of the 0.9% saline followed by the intra-arterial administration of SP as a control (p < 0.05).
Iontophoretic application of the NK1 receptor antagonists (0.2~3.4 mM) following the intra-arterial administration of SP resulted in less increase of PBF than the iontophoretic application of the 0.9% saline following the intra-arterial administration of SP as a control (p < 0.05).
Therefore, the results of the present study provide evidences that the iontophoretic application is an effective method to deliver drugs to the dental pulp, and that iontophoretically applied NK1 receptor antagonists block SP-induced vasodilation effectively. The above results show the possibility that the iontophoretical application of NK1 receptor antagonists can control the neurogenic inflammation in the dental pulp.
Pulpal inflammation can result from mechanical, chemical, or bacterial insult. The initial vascular reactions during inflammation in the dental pulp are vasodilation and increased vascular permeability. These vascular reactions are induced by activation of sensory nerve endings of C-fiber. This condition is called neurogenic inflammation1). The inflammatory process in the pulp does not differ significantly from that in other tissues in most respects with a notable exception, the low compliance environment2). Activation of sensory nerve causes release of vasoactive peptides from its endings. These neuropeptides include substance P (SP), neurokinin A, and calcitonin gene-related peptide. Sensory neuropeptides initiate release of inflammatory agents such as histamine, prostaglandins, leukotrienes and bradykinin3). The release of inflammatory mediators results in vasodilating arterioles, venular leakage, leukocytes chemotaxis, and pain induction2).
SP, discovered in 1931 from alcoholic extracts of horse brain and intestine4), is widely found in the central and peripheral nervous system. It is a very small peptide that is composed of 10 amino acids. Dental pulp is known to have the highest concentration of SP outside of central nerve system5). Existence of SP like-immunoreactivity was confirmed in the dental pulp6).
SP-containing primary neurons perform two functions: the transmission of sensory signals to second-order neurons on the central side; and the control of a variety of activities on the peripheral side, such as vasodilation, plasma extravasation, contraction of smooth muscles, acceleration of mucociliary movements, and depolarization of peripheral neurons7). Therefore it is known to play an important part in vasoregulation: it is a strong vasodilator and increasing vascular permeability6,8) and also involved in nociception (pain) associated with inflammation9).
SP showed to exert flow response on the dental pulp8). With the use of electrical stimulation, the vasodilation in the pulp was proposed to be caused by liberation of sensory neuropeptides6). Endothelium dependant NO pathway was shown to be involved in this vasodilatory function of SP in the dental pulp10). There is also evidence that SP is intermittently released from nerves without any apparent stimulation. Role of such physiological intermittent leakage of the neuropeptide is vasodilation that modulates sympathetic vasoconstriction during resting basal condition. Resting vasodilator tone due to release of SP was suggested to exist in the ferret dental pulp11).
SP is also a pain transmitter of certain primary sensory neuron producing slow excitatory postsynaptic potentials in second order neuron in the dorsal horn12). Various inflammatory mediators including SP causes pain directly by lowering the excitability threshold, and indirectly by vasodilating arterioles, vascular leakage in venule resulting in edema and subsequent elevation in tissue pressure.
SP belongs to the tachykinin group, a family of related peptides sharing the common carboxyl terminus, Phe-X-Gly-Leu-Met-NH213) and its roles are performed through its preferential interaction with the NK1 receptor14). There are three major mammalian tachykinins: SP, neurokinins A (NKA) and B (NKB)15). When released by appropriate stimuli in peripheral organs, these neuropeptides activate receptor types, NK1, NK2, and NK3. Of the natural agonists, SP has the highest affinity for NK1, NKA for NK2, and NKB for NK316). NK1 agonist is already identified as SP and it is one of the most important neuropeptide involved in the hemodynamics and neurogenic inflammation in the dental pulp17).
SP antagonists have been used in studies to define the exact physiological and pathological roles of SP and to control the SP-induced phenomenon. Studies for the roles of SP include those for resting interstitial fluid pressure11), for the basal blood flow of pulp and gingiva18), and for the function of SP in pain transmission and the vascular system in various organs of animals9), and NK1 receptor antagonists were characterized17). Studies for the control of SP-induced responses include those in blocking the SP-induced responses such as the change in the blood flow, tissue pressure and vascular permeability11), its associated jaw-opening reflex19-21), in blocking responses elicited by noxious stimulus9,22). These NK1 receptor antagonists has been shown to be effective in blocking peripheral inflammatory vascular responses as well as nociception (pain) associated inflammation. Hence, NK1 receptor antagonist is expected to provide novel therapies for important diseases where SP is involved23).
In these previous studies, most of NK1 receptor antagonists were administered intra-arterially, intra-venously, or intra-thecally for investigating its effects on pulpal blood flow (PBF). However, the effect of local application of NK1 receptor antagonists into dentinal cavity was not defined yet. It needs to be evaluated whether iontophoretically applied NK1 receptor antagonists can control the pulpal inflammation. Therefore, the purpose of this study was to investigate the influence of NK1 receptor antagonists on the PBF when they were applied iontophoretically through the dentinal cavity of teeth.
Eleven cats with average weight of 3.4 kg were used. Periapical radiographs were taken of the canine teeth to ensure the maturity of the apices and to assess the size of the pulp. Animals were initially anesthetized with intra-muscular injection of ketamine (75 mg/kg) and acepromazine (2.5 mg/kg), followed by intra-venous injection of alpha-chloralose (40 mg/kg) and urethane (500 mg/kg) through the femoral vein. General anesthesia was maintained with supplemental anesthetics (4 mg/kg of alpha-chloralose and 50 mg/kg of urethane) as needed. Airway was maintained through the tracheostomy and a femoral artery was cannulated for monitoring systemic blood pressure. A lingual artery was catheterized for the administration of drugs into the maxillary artery. The tip of the catheter was positioned at the junction of the external carotid and lingual arteries to insure unimpeded blood flow of the external carotid artery. Body temperature was monitored with rectal thermometer and maintained between 36℃ and 39℃ with a heating pad. The mandible was immobilized by intermaxillary splinting with dental stone and a steel rod that was anchored to a base by magnetic stand.
Enamel was removed with a dental bur on the distal surface of the crown over the cervical third of the canine teeth ipsilateral to the catheterized lingual artery, which was aparted from gingival margin more than 3 mm. The laser Doppler flowmeter probe (PF416, Perimed Co., Stockholm, Sweden) was carefully positioned to record PBF through the exposed dentin, which was flooded with isotonic saline to avoid drying. The PBF was monitored with a laser Doppler flowmetry (Periflux 4001, Perimed Co., Stockholm, Sweden). Systemic blood pressure recording (in mmHg) and the laser Doppler recording of PBF (in perfusion unit) were monitored continuously and simultaneously throughout the experiments through the computer monitor with a physiological pressure transducer (Harvard Apparatus Ltd., Edenbridge, Kent, England), Digidata 1200, and Axoscope (Axon Instruments, Inc, Foster City, CA, USA).
A lower canine ipsilateral to the catheterized lingual artery was prepared for iontophoresis. A class V dentinal cavity (1.5 mm in width, 2 mm in length, 0.5 mm in depth) was prepared on the cervical third of the labial surface of the crown with a high-speed #701 tapered fissure bur. The exposed dentin surface was etched with 32% phosphoric acid for 10 sec so that it was cleaned of debris and the dentinal tubules were open. The iontophoretic electrode (PeriIont Micropharmacology System, Perimed, Jarfallo, Sweden) was placed on the tooth cavity and reference electrode was placed on the adjacent soft tissue. Drug was delivered using a cathodal current with 0.02~0.1 mA for 1 min. The current was calibrated at the intensity and duration on which iontophoretic application of saline does not alter PBF.
SP (Sigma Chem. Co., St. Louis, MO, USA) was injected into the lingual artery at doses of 0.8~20.0 ng/kg 15 sec before or after iontophoretic application of NK1 receptor antagonists. As a control, 0.2 mL of normal saline solution was injected into the lingual artery. The selected drugs were given into the maxillary artery for 40 sec, followed by a flush of 0.3 mL isotonic saline. SP was titrated to exert maximum effect on PBF without influencing systemic blood pressure in order to minimize or eliminate the systemic effect. To compare the effects of drugs on the pulp with and without iontophoresis, drugs were applied to the dentinal cavity with and without iontophoresis. In order to confirm the iontophoresis function in each tooth cavity, SP was applied iontophoretically in each animal at doses of 59.4~742.1 µM before other drugs. For NK1 receptor antagonist, two kinds of peptide were used: [D-Pro2, D-Trp7,9]-SP and [D-Pro2, D-Phe7, D-Trp9]-SP (Sigma Chem. Co., St. Louis, MO, USA)17). These NK1 receptor antagonists were applied iontophoretically into the dentinal cavity at doses of 0.2~3.4 mM. NK1 receptor antagonists were titrated to exert maximum inhibitory effect on PBF without influencing systemic blood pressure. As a control, normal saline solution was applied iontophoretically into the dentinal cavity. Effect of NK1 receptor antagonists on PBF was tested in two ways: 1) to identify the influence of NK1 receptor antagonists on PBF change induced by SP, NK1 receptor antagonists were applied iontophoretically into the tooth cavity 15 sec after the start of intra-arterial administration of SP 2) to identify the influence of NK1 receptor antagonists on PBF change which may be induced by SP, NK1 receptor antagonists were applied iontophoretically into the tooth cavity 15 sec before the start of intra-arterial administration of SP. A stable laser Doppler signal during the recording of 40 sec before each administration of drugs was defined, and the maximum deviation after each administration was used as the experimental value. The PBF values were recorded in the computer until laser Doppler flowmeter readings returned to the control level.
All numerical data in the text and tables are expressed as percent change from control and are given as mean ± standard error of the mean (SEM). The paired variables of control and experimental data were compared by paired t-test and differences with p < 0.05 were considered statistically significant.
A typical strip-chart recording of systemic blood pressure and PBF in response to intra-arterial administration of SP is presented in Figure 1. Percentage changes of PBF in response to SP and saline as control are given in Figure 2. Intra-arterial injection of SP at the dose of 0.8~20.0 ng/kg resulted in increases of PBF by 38.9 ± 4.2% (n = 35, p < 0.05), whereas there was no significant change of PBF with the physiologic saline as a control.
Percentage changes of PBF to the dentinal application of the drugs are given in Figure 3. Dentinal application of SP and SP antagonist without iontophoresis caused little effect on PBF. However, iontophoretic application of SP at the dose of 59.4~742.1 µM and SP antagonists at the dose of 0.2~3.4 mM resulted in PBF increase by 17.5 ± 3.1% (n = 19) and 7.0 ± 6.3% (n = 9) respectively, both of which were significantly different from those without iontophoresis (p < 0.05).
Percentage changes of PBF in response to iontophoretic application of SP antagonists before intra-arterial administration of SP and saline as control are given in Figure 4. Iontophoretic application of SP antagonists at dose of 0.2~3.4 mM before intra-arterial administration of SP at dose of 0.8~20.0 ng/kg resulted in increase of PBF by 10.5 ± 3.1% (n = 10) and 26.1 ± 8.0% (n = 6) in control. There was significant difference between SP antagonists and control (p < 0.05). As a result, SP antagonists effectively blocked PBF increase caused by SP.
A typical strip-chart recording of systemic blood pressure and PBF in response to the iontophoretic application of SP antagonists after intra-arterial administration of SP on PBF is presented in Figure 5. Percentage changes of PBF in response to iontophoretic application of SP antagonists after intra-arterial administration of SP and saline as control are given in Figure 6. Iontophoretic application of SP antagonists at dose of 0.2~3.4 mM after intra-arterial administration of SP at dose of 0.8~20.0 ng/kg resulted in increase of PBF by 11.0 ± 2.8% (n = 19) and 25.0 ± 3.8% (n = 19) in control. There was significant difference between SP antagonists and control (p < 0.05). As a result, SP antagonists effectively attenuated SP-inducing PBF increase.
In neurogenic inflammation, vasodilation and increased vascular permeability is resulted from the release of neuropeptides from sensory nerve endings1,24-29). It is widely known that SP plays important roles in neurogenic inflammation. SP causes pain directly by lowering the excitability threshold of nerve and indirectly by elevated tissue pressure through vascular change in inflammation2). SP also caused enhanced vasodilation in the cutaneous circulation30,31). By Lembeck et al.30), infusion of SP into the femoral artery resulted in dose-dependant vasodilation and plasma extravasation. Vasodilation and plasma extravasation following antidromic stimulation of sensory nerves are initiated by peripheral release of SP from chemosensitive pain fibers. In the study of Louis et al.31), topical application of mustard oil caused an increase of blood flow in rat hind paw and this increase was significantly decreased by SP antibodies. These results suggest that SP was involved in mediating neurogenic inflammatory responses.
SP has been shown to increase blood flow in dental pulp. In the study of Olgart et al.32), SP was given as an intra-venous bolus to investigate the enhanced vasodilation in sensory denervated tooth pulps by SP. SP injection caused dose-dependant increases in PBF of unilaterally denervated cats and the magnitude of the responses was several folds larger than that of control side. In the study of Kim et al.17), intra-arterial injection of NK1 receptor agonist, [Sarcosine]-SP, resulted in significant increase of PBF in a dose-dependant manner. The same result was shown in the study of Kim et al.10). This vasodilatory function of SP in the dental pulp was confirmed in the present study and in the previous studies10,17,32) and this finding supports that SP plays an important role of vasodilation in neurogenic inflammation.
In the present study, intra-arterial administration of SP resulted in initial increase followed by a rapid decrease of PBF. These findings may be due to the low compliance environment of dental pulp. Initial vasodilation of pulpal arterioles leads to an increase in pulpal interstitial pressure, which leads to a collapse of pulpal venules33-35). By the study of Kim et al.8), immediately after SP injection, PBF increased significantly, which was followed by decrease after 30 sec of SP injection. The same phenomenon was shown in the present study. Basically three types of PBF responses to drugs have been explained33). In Type I, PBF decrease markedly with the intra-arterial administration of norepinephrine35) or 5-hydroxytriptamine36), with electrical stimulation of the cervical sympathetic nerve and reflex excitation of the sympathetic nervous system by hemorrhage and extreme hematocrit variations33,37). This type of flow response is due to activation of α-receptors located in pulpal resistance vessels and the activation of sympathetic adrenergic vasoconstrictor fibers33,34). In the type II response, PBF decreases gradually, as after the intra-arterial infusion of histamine. This is probably due to an increase in capillary permeability and resultant increased tissue pressure in the low compliance system of the dental pulp. In the type III response, an initial increase is followed by a rapid decrease. This unusual biphasic response is caused by SP, isoproterenol, prostaglandin E2, and bradykinin33,35,38) and is a result of the low compliance environment of the pulp. In a low compliance environment of dental pulp, due to lack of distensibility, any gain in volume must necessarily increase the pulpal hydrostatic tissue pressure. If the tissue pressure rises to the level of intravascular pressure it will compress soft-walled venule, thus counteracting any beneficial blood flow increase during pulpal inflammation1). In the present study, PBF was decreased after PBF increase when SP was administered iontophoretically. Explanation of this phenomenon may be possible sympathetic nerve stimulation by iontophoresis. Because decreased PBF was recovered to control level when the iontophoretic application was finished, there is some possibility of the intradental sympathetic nerve activation during the iontophoresis.
NK1 receptor antagonists have been used to control the SP-induced responses and showed significant effect. Intra-oral administration of RPR 100893 showed to have some usefulness in blocking the pain and its associated jaw-opening reflex in guinea pigs19), and Galanin blocked the facilitation of the flexor reflex induced by C-fiber CS (a brief conditioning electrical stimulus train)20). Spantide II antagonized the facilitation of the flex reflex induced by intra-thecal SP as well as by C-fiber CS21). CP-96,345 blocked responses elicited by noxious thermal stimulus9) and intra-venous administration of it blocked the potential evoked by tooth pulp stimulation in superficial layers of the trigeminal nucleus caudalis in rabbits22). In the tooth, close intra-arterial infusion of NK1 receptor antagonists blocked the SP-induced responses such as the change in the blood flow, tissue pressure and vascular permeability in ferret11), and close intra-arterial injection of [D-Pro2, D-Trp7,9]-SP, [D-Pro2, D-Phe7, D-Trp9]-SP, CP-96, 345, and RP-67, 580 blocked effectively SP-induced PBF increase in cat17).
There are two kinds of NK1 antagonists: peptide antagonist and non-peptide one. In the present study, two kinds of NK1 receptor antagonists were used, which are [D-Pro2, D-Trp7,9]-SP and [D-Pro2, D-Phe7, D-Trp9]-SP. Two aspects of the antagonists can be considered. One is change in PBF by antagonists themselves; the other is the antagonistic effect of the drugs on SP function. To be a specific pharmacological tool for any receptor characterization, the antagonist needs to have no or minor effect on physiological function when it is administered alone17), and it should effectively block the agonistic effect on physiological function. The design of clinically useful SP antagonists will probably therefore require not only improvement in antagonistic potency but also elimination of agonistic activity39). In the study of Kim et al.17), two kinds of peptide antagonist, [D-Pro2, D-Trp7,9]-SP and [D-Pro2, D-Phe7, D-Trp9]-SP and two kinds of non-peptide antagonists, CP-96,345 and RP-67, 580 were evaluated. The antagonist [D-Pro2, D-Trp7,9]-SP effectively attenuated PBF increase caused by SP without causing any significant changes in either systemic arterial blood pressure or PBF. The antagonists [D-Pro2, D-Phe7, D-Trp9]-SP, CP-96,345 and RP-67,580 also effectively attenuated PBF increases caused by SP. However, when injected alone, [D-Pro2, D-Phe7, D-Trp9]-SP, CP-96,345, and RP-67,580 caused significant increases in PBF. Therefore, [D-Pro2, D-Trp7,9]-SP was suggested to be the more suitable antagonist for the study of SP and NK1 receptor-related hemodynamic studies in the feline dental pulp17). Use of potent selective and bioavailable antagonists can help to define the exact physiological and pathological role of SP19).
In the present study, intra-arterial and iontophoretic applications were used to deliver drugs to the dental pulp. Local application of various agents to the dental pulp needs access to or close to the dental pulp. It requires deep preparation into the dentin and entails the risk of damage to the pulp, which are invasive. Deep preparation in cat dentin showed both direct effects on PBF and indirect effects via excitation of pulpal nerves25). On the contrary, iontophoresis is an alternative non-invasive method that enables pharmacological agents to selectively reach the pulp without changing the normal conditions of the tissue, and it should not cause any systemic effects obscuring the true pulpal reactions40). Iontophoretic current per se caused no or minor vasodilator effects when the current intensity was below 100 µA. Sympathetic vasoconstriction was obtained with all current intensities in a current-dependant manner. The current threshold for iontophoretic delivery of various drugs were 20~100 µA in the previous studies. In the present study, cathodal current was used with the same 0.02~0.1 mA and this current was calibrated on which iontophoretic delivery of saline do not alter PBF. When the effect on the dental pulp was compared between with and without the use of iontophoresis in the present study, iontophoretic application of SP induced a significant increase in PBF while simple dentinal application of SP without iontophoresis showed non-significant increase in PBF. This insignificant influence of the simple application on PBF may be due to the dentinal resistance with continuous outward movement of dentinal fluid through exposed dentine41). In the study of Vongsavan et al.29), chemical compounds such as Evans blue, Janus green and methylene green did not penetrate into the vital pulp through shallow dentinal cavities. Therefore, iontophoresis was shown to be an effective method to deliver drugs to the dental pulp without deep dentin preparation or pulpal damage.
To evaluate the vascular effect of iontophoretically applied drugs on the dental pulp, a non-invasive monitoring technique, the laser Doppler flowmeter assessment was used in the present study. Laser Doppler flowmeter assessments of iontophoretically applied vasoactive substances were performed at different body areas in some previous studies which include the effects of norepinephrine on human finger skin circulation42), acetylcholine on human foot skin43,44), acetylcholine and sodium nitrite on human foot skin45), acetylcholine and sodium nitrite on human forearm skin46), and acetylcholine and nitroprusside on human foot skin47).
The effect of iontophoretical application of NK1 receptor antagonists was evaluated in two ways in the present study. One was focused on the control of SP-induced vasodilation, and the other was on the prevention of SP-inducing vasodilation. To evaluate the effect of NK1 receptor antagonist in controlling SP-induced PBF increase, NK1 receptor antagonists were administered iontophoretically after intra-arterial administration of SP in the present study, which resulted in the less increase of SP-induced PBF than control. This finding provides evidence that iontophoretically applied NK1 receptor antagonist can control the SP-induced PBF increase in certain degree. To evaluate the effect of NK1 receptor antagonist in preventing SP-inducing PBF increase, NK1 receptor antagonists were administered iontophoretically before intra-arterial administration of SP in the present study, which resulted in the less increase of SP-induced PBF than control. This finding provides evidence that iontophoretically applied NK1 receptor antagonist can prevent SP-induced PBF increase in certain degree. These findings in the present study using iontophoresis showed similar findings with those studies that used intra-oral or intra-arterial administration of NK1 receptor antagonists in that NK1 receptor antagonist effectively inhibited SP-induced responses.
Therefore, the results of the present study provide evidences that the iontophoresis is an effective method to deliver drugs to the dental pulp, and that iontophoretically applied NK1 receptor antagonists block SP-induced vasodilation effectively. The above results show the possibility of iontophoretically applied NK1 receptor antagonists in the control of the neurogenic inflammation in the dental pulp.
SP has been implicated in peripheral inflammatory responses and recent evidences from many researches indicate that NK1 receptor antagonists are effective in blocking peripheral inflammatory responses as well as nociception associated with inflammation9). Since the same NK1 receptor antagonists as in the present study, [D-Pro2, D-Trp7,9]-SP and [D-Pro2, D-Phe7, D-Trp9]-SP, blocked not only vasodilatory function of SP but also pain transmission when administered intra-arterially48,49), iontophoretic application of these antagonists through the dentinal cavity also may inhibit the pulpal pain too. Therefore, judging from the results of previous studies and present study, it can be assumed that NK1 receptor antagonists will be used in regulation of pulpal inflammation. Further research is necessary to evaluate the iontophoretic application of NK1 receptor antagonists in human teeth.
Figures and Tables
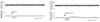 | Figure 1Changes in pulpal blood flow in response to the intra-arterial (i.a.) administration of normal physiologic saline (A) and substance P (5.0 ng/kg) (B). |
 | Figure 2Changes in pulpal blood flow in response to the intra-arterial (i.a.) administration of the substance P (SP) (0.8~20.0 ng/kg) (mean±SEM). *Significantly different at p < 0.05. |
 | Figure 3Changes in pulpal blood flow in response to dentinal application of the substance P (SP) (59.4~742.1 µM) and its antagonists (Anta.) (0.2~3.4 mM) with (w/) and without (w/o) iontophoresis (mean ± SEM). *Significantly different at p < 0.05. |
 | Figure 4Changes in pulpal blood flow in response to the iontophoretic (i.t.p.) application of the substance P (SP) antagonists (Anta.) (0.2~3.4 mM) followed by intra-arterial (i.a.) administration of SP (0.8~20 ng/kg) (mean ± SEM). *Significantly different at p < 0.05. |
References
1. Heyeraas KJ, Kvinnsland I. Tissue pressure and blood flow in pulpal inflammation. Proc Finn Dent Soc. 1992. 88:Suppl 1. 393–401.
2. Kim S. Neurovascular interactions in the dental pulp in health and inflammation. J Endod. 1990. 16(2):48–53.

4. V Euler US, Gaddum JH. An unidentified depressor substance in certain tissue extracts. J Physiol. 1931. 72:74–87.

5. Olgart L, Gazelius B, Brodin E, Nilsson G. Release of substance P-like immunoreactivity from the Dental Pulp. Acta Physiol Scand. 1977. 101:510–512.

6. Heyeraas KJ, Kim S, Raab WHM, Byers MR, Liu M. Effect of electrical tooth stimulation on blood flow, interstitial fluid pressure and substance P and CGRP-immunoreactive nerve fibers in the low compliant cat dental pulp. Microvasc Res. 1994. 47:329–343.

7. Lembeck F, Gamse R. Substance P in Peripheral Sensory Processes. Ciba Found Symp. 1982. (91):35–54.

8. Kim S, Dorscher-Kim JE, Liu MT, Trowbridge HO. Biphasic pulp blood-flow response to substance P in the dog as measured with a radiolabelled, microsphere injection method. Arch Oral Biol. 1988. 33:305–309.

9. Henry JL. Substance P and inflammatory pain: potential of substance P antagonists as analgesics. Agents Actions Suppl. 1993. 41:75–87.
10. Kim SK, Karabucak B, Welsch H, Simchon S, Kim S. Intracellular mechanism of substance P-induced vasodilatation in bovine dental pulp. J Endod. 2001. 27:231.
11. Berggreen E, Heyeraas KJ. The role of sensory neuropeptides and nitric oxide on pulpal blood flow and tissue pressure in the ferret. J Dent Res. 1999. 78(9):1535–1543.

12. Otsuka M, Yanagisawa M. Does substance P act as a pain transmitter? Trends Pharmacol Sci. 1987. 8:506–510.

13. Buck SH, Burcher E. The tachykinins: a family of peptides with a brood of 'receptors'. Trends Pharmacol Sci. 1986. 7:65–68.

14. Watling KJ, Krause JE. The rising sun shines on substance P and related peptides. Trends Pharmacol Sci. 1993. 14:81–84.

15. Hokfelt T, Pernow B, Wahren J. Substance P: a pioneer amongst neuropeptides. J Intern Med. 2001. 249:27–40.

16. Regoli D, Drapeau G, Dion S, Couture R. New selective agonist for neurokinin receptors: pharmacological tools for receptor characterization. Trends Pharmacol Sci. 1988. 9:290–295.

17. Kim SK, Ang L, Hsu YY, Kim S. Characterization of NK1 receptor antagonists on pulpal hemodynamics in cats. J Endod. 1995. 21(4):229.

18. Berggreen E, Heyeraas KJ. Effect of the sensory neuropeptide antagonists h-CGRP((8-37)) and SR 140.33 on pulpal and gingival blood flow in ferrets. Arch Oral Biol. 2000. 45:537–542.

19. Alia S, Azérad J, Pollin B. Effects of RPR 100893, a potent NK1 antagonist, on the jaw-opening reflex in the guinea pig. Brain Res. 1998. 787(1):99–106.

20. Wiesenfeld-Hallin Z, Villar MJ, Hokfelt T. The effects of intrathecal galanin and C-fiber stimulation on the flexor reflex in the rat. Brain Res. 1989. 486:205–213.

21. Wiesenfeld-Hallin Z, Xu XJ, Håkanson R, Feng DM, Forker T. The specific antagonistic effect of intrathecal Spantide II on substance P and C-fiber conditioning stimulation-induced facilitation on the nociceptive flexor reflex in the rat. Brain Res. 1990. 526:284–290.

22. Yonehara N, Sawada T, Matsuura H, Inoki R. Influence of electro-acupuncture on the release of substance P and the potential evoked by tooth pulp stimulation in the trigeminal nucleus caudalis of the rabbit. Neurosci Lett. 1992. 142(1):53–56.

23. Beattie DT, Connor HE, Hagan RM. Recent development in tachykinin NK1 receptor antagonists: prospects for the treatment of migraine headache. Can J Physiol Pharmacol. 1995. 73:871–877.

24. Kim S, Liu M, Simchon S, Dörscher-Kim JE. Effects of selected inflammatory mediators on blood flow and vascular permeability in the dental pulp. Proc Finn Dent Soc. 1992. 88:Suppl 1. 387–392.
25. Olgart L, Edwall L, Gazelius B. Involvement of afferent nerves in pulpal blood-flow reactions in response to clinical and experimental procedures in the cat. Arch Oral Biol. 1991. 36:575–581.

26. Liu M, Pertl C, Markowitz K, Dörscher-Kim JE, Kim S. The effects of capsaicin on pulpal blood flow. Proc Finn Dent Soc. 1992. 88:Suppl 1. 463–467.
27. Raab WH. Temperature related changes in pulpal microcirculation. Proc Finn Dent Soc. 1992. 88:Suppl 1. 469–479.
28. Fazekas A, Gyorfi A, Irmes F, Rosivall L. Effect of substance P administration on vascular permeability in the rat dental pulp and submandibular gland. Proc Finn Dent Soc. 1992. 88:481–486.
29. Vongsavan N, Matthews B. Changes in pulpal blood flow and in fluid flow through dentine produced by autonomic and sensory nerve stimulation in the cat. Proc Finn Dent Soc. 1992. 88:Suppl 1. 491–497.
30. Lembeck F, Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979. 310:175–183.

31. Louis SM, Jamieson A, Russell NJW, Dockray GJ. The role of substance P and calcitonin gene-related peptide in neurogenic plasma extravasation and vasodilatation in the rat. Neuroscience. 1989. 32(3):581–586.

32. Olgart L, Gazelius B. Enhanced vasodilation in sensory denervated tooth pulp by substance P (SP). Reg Pep. 1988. 22:(Abstract) 137.
35. Kim S, Dörscher-Kim JE. Hemodynamic regulation of the dental pulp in a low compliance environment. J Endod. 1989. 15(9):404–408.

36. Kim S, Trowbridge H, Dörscher-Kim JE. The effects of 5-hydroxytryptamine on pulpal hemodynamics in dogs. J Dent Res. 1986. 65:682–685.
37. Kim S, Fan FC, Chen RYZ, Simchon S, Schuessler GB, Chien S. Symposium: 3. Effects of changes in systemic hemodynamic parameters in pulpal hemodynamics. J Endod. 1980. 6:394–399.

38. Kim S. Regulation of blood flow of the dental pulp: macrocirculation and microcirculation studies [Ph. D. dissertation]. 1981. New York, NY: Columbia University.
39. Håkanson R, Leander S, Andersson RG, Hörig J. Substance P antagonists release histamine from peritoneal mast cells. Acta Physiol Scand. 1983. 117:319–320.

40. Kostouros GD. Iontophoretic delivery of pharmacological agents to the dental pulp, an experimental study in the rat. 1996. Stockholm, Sweden: Department of Physiology and Pharmacology, Karolinska Institutet;1–39.
41. Vongsavan N, Matthews B. The permeability of cat dentine in vivo and in vitro. Arch Oral Biol. 1991. 36:641–646.

42. Lindblad LE, Ekenvall L, Ancker K, Rohman H, Oberg PA. Laser Doppler flow-meter assessment of iontophoretically applied norepinephrine on human finger skin circulation. J Invest Dermatol. 1986. 87:634–636.

43. Walmsley D, Wiles PG. Early loss of neurogenic inflammation in the human diabetic foot. Clin Sci (Lond). 1991. 80:605–610.

44. Parkhouse N, Lequesne PM. Quantitative objective assessment of peripheral nociceptive C fibre function. J Neurol Neurosurg Psychiatry. 1988. 51:28–34.

45. Westerman RA, Widdop RE, Hogan C, Zimmet P. Non-invasive tests of neurovascular function: reduced responses in diabetes mellitus. Neurosci Lett. 1987. 81:177–182.

46. Roberts RG, Westerman RA, Widdop RE, Kotzmann RR, Payne R. Effects of capsaicin on cutaneous vasodilator responses in humans. Agents Actions. 1992. 37:53–59.

47. Westerman RA, Widdop RE, Hannaford J, Low A, Roberts RG, Kent P, Sideris K, Yip T, Hales JR, Stephens FR. Laser Doppler velocimetry in the measurement of neurovascular function. Australas Phys Eng Sci Med. 1988. 11:53–66.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download