Abstract
This study evaluated the microshear bond strength of composte resin to teeth bleached with commercial whitening strips and compared with those bleached with home bleaching gel. Twelve extracted human central incisors were cut into pieces and central four segments were chosen from each tooth and embedded in acrylic resin. Four blocks with 12 tooth segments embedded in acrylic resin were acquired and numbered from group one to group four. Group 1 was bleached with Crest Whitestrips, group 2 with Claren, group 3 with Opalescence tooth whitening gel (10% carbamide peroxide). Group 4 was used as control. The bleaching procedure was conducted for 14 days according to the manufacturer's instructions; the bleaching strips twice a day for 30 min and the bleaching gel once a day for 2 hr. After bleaching, composite resin (Filtek Supreme) was bonded to the enamel surfaces with a self-etching adhesive (Adper Prompt L-Pop) using Tygon tube. Microshear bond strength was tested with a universal testing machine (EZ-test). The data were statistically analysed by one-way ANOVA. The study resulted in no statistical differences in microshear bond strength between the tooth segments bleached with 2 different whitening strips and bleaching gel. It can be concluded that the effect of bleaching with either commercial whitening strips or bleaching gel on enamel is minimal in bonding with self-etching adhesive to composite resin.
Vital teeth tend to present color changes that compromise esthetics substantially. These discolorations can be extrinsic or intrinsic according to the location and the cause of the stain. Many dentists have indicated the treatment of intrinsic discolorations with bleaching agents1). Thanks to innovations in bleaching agents, tooth whitening has become more and more popular among dental professionals and patients2). The most common treatment for intrinsic discoloration is bleaching with hydrogen peroxide, which includes professionally administered (in office), professionally dispensed (custom tray based) and self directed (over-the-counter) bleaching3). For in office bleaching, 30 to 35% hydrogen peroxide was used and monitoring was required by the dentists4). Therefore the professionally dispensed bleaching, also called nightguard vital tooth bleaching became popular due to its simplicity. This method was first described and published by Haywood and Heymann5) and 10 to 15% carbamide peroxide was used in a custom made mouthguard for several weeks. Nowadays, these systems use 10% carbamide peroxide with carboxypolymethylene polymer as a thickening agent for improvement of tissue adherence and for sustained release of the whitening agent. In the original protocol, the average time recommended for optimal color change was 6 weeks, although slight whitening effects may be noted as early as 2 weeks.
Recently, newly developed whitening strips were introduced, which use flexible polyethylene strips to deliver hydrogen peroxide bleaching gel to the dentition. Considering the overall peroxide dose, contact time, and the ease of use, this trayless delivery system is said to be more advantageous than the other delivery systems3).
The bleaching effect of whitening strips is known to be close to the custom tray based bleaching. The study of Donly et al.3) compared the responses of whitening strips and custom tray bleaching with 10% carbamide peroxide in children with tetracycline stain and showed highly significant color improvements for both systems.
When the bleached teeth were not harmonic with the adjacent teeth due to insufficient whitening effect, the teeth must be restored with composite resin or veneer crown. As the bleaching of vital teeth involves direct contact of the whitening agents on the outer enamel surface for an extended period of time, studies have demonstrated the potential adverse effects of these carbamide peroxide agents2). However, the effect of pre-restorative carbamide peroxide bleaching on the bond strength of composite resin to enamel were inconclusive. Some authors reported severe decrease in the average bond strength of composite resin to bleached enamel compared with unbleached enamel, while other studies indicated means to counteract adverse bleaching effects and showed no statistical differences6).
As whitening strips are being used more and more commonly at home, teeth bleached with these bleaching strips have the possibility of being treated with composite resin restoration without notice. However, there were no previous studies on the bond strength of composite resin to enamel bleached with commercially available whitening strips.
The purpose of this study was to evaluate the effects of the commercially available whitening strips on microshear bond strength of composite resin to enamel and to compare those with the effect of the 10% carbamide peroxide bleaching agents.
Two commercially available whitening strips and one bleaching gel were used in this study: Crest Whitestrips (The Procter & Gamble Company, Cincinnati, USA), Claren dental whitening solution (LG Household & Health Care, Seoul, Korea) and Opalescence tooth whitening gel (Ultradent Product Inc, South Jordan, USA). Crest Whitestrips consists of flexible polyethylene strip that is coated with an adhesive hydrogen peroxide bleaching gel. The strips carry 150 to 200 mg of whitening gel distributed uniformly across the strip surface. The hydrogen peroxide concentration of whitening strip is 5.3%3). Claren dental whitening solution has the hydrogen peroxide concentration of 2.6%. The Opalescence bleaching gel used in nightguard bleaching is composed of 10% carbamide peroxide with carbopol.
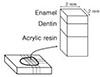
Twelve freshly extracted human central incisors with flat labial surfaces were chosen for this study. The tooth structure palatal to the pulp space was cut off with high speed diamond bur to prohibit the tooth segment from separating at the pulp space after sectioning, and to enhance the bonding of the tooth to acrylic resin. The teeth without palatal surfaces were then embedded into acrylic resin with the labial surface exposed in a rectangular form for convenience of mounting to the precision cutting device (Accutom-50, Struers, Copenhagen, Denmark). The acrylic resin specimen was fixed on the vice, and sectioned serially to a 2×2 mm square form with a diamond cutting wheel (Diamond cut-off wheel, Struers, Copenhagen, Denmark). The most central 4 segments per teeth were chosen to execute the study (Figure 1). Finally, 48 (12 × 4) tooth specimens were obtained. Four segments from each tooth were divided into 4 groups randomly and embedded into acrylic resin to form a block. As a result, 4 blocks were fabricated for this study.
Group 1 (Opal) was bleached with Opalescence tooth whitening gel with 10% carbamide peroxide. Group 2 (Cla) was bleached with Claren dental whitening solution and group 3 (Cre) with Crest Whitestrips. Group 4 (Con) was used as a control. Groups 1, 2, 3 were bleached with corresponding bleaching agents according to the manufacturer's instructions. In group 1, Opalescence tooth whitening gel was applied for 2 hours per day. The gel was applied over the entire enamel surface approximately to 0.5 mm thickness. During bleaching, the block was stored in the incubator of 100% humidity at 37℃. After 2 hours bleaching, the gel was washed away with tap water for 30 seconds. Groups 2 and 3 were bleached with corresponding whitening strips for 30 minutes twice a day. The bleaching interval was 12 hours. During bleaching, they were stored in artificial saliva at 37℃ in the incubator. The artificial saliva consists of 50% NaCMC, 10% glycerine and 40% tertiary distilled water. After bleaching, the whitening strips were removed and cleaned with tap water for 30 seconds. The blocks were stored in an artificial saliva at 37℃ in the incubator with the exception of the bleaching time. In this way, groups 1, 2 and 3 were bleached for 14 days. Group 4, the control, was stored in the artificial saliva for 14 days without any treatment mentioned previously. The test blocks were then stored in artificial saliva at 37℃ in the incubator for 24 hours before composite resin bonding.
For composite resin bonding, self-etching adhesive (Adper Prompt L-Pop, 3M ESPE, St Paul, USA) was applied on the bleached enamel surface of the exposed tooth segment (Table 1). After 15 seconds, the excess bonding agent was removed with the air stream of the 3-way syringe and light cured (Elipar Freelight 2, 3M ESPE, St Paul, USA) for 10 seconds. Then composite resin (Filtek Supreme, 3M ESPE, St Paul, USA) was filled into the Tygon tube (TYG-030, Small Parts Inc., Miami Lakes, USA) with an internal diameter of approximately 0.7 mm, which was cut approximately to 2 mm in length. The Tygon tube was held tightly to prevent the resin from seeping away from the defined area at the enamel surface. The composite resin was light cured for 20 seconds through the transparent Tygon tube. In this manner, small cylinders of composite resin, approximately 0.7 mm in diameter and 2 mm in height, were bonded to small enamel surfaces of approximately 0.4 mm2. Subsequently, the Tygon tube was removed to carry out the microshear bond strength test.
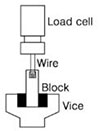
The blocks containing composite resin cylinders attached on the tooth segments were fixed in the vice of a universal testing machine (Ez-test, Shimadzu Co, Kyoto, Japan). A fine ligature wire (0.2 mm in diameter) that was almost touching the enamel surface, was looped around the composite resin cylinder. The composite resin cylinder, the wire and the center of the load cell were aligned as straight as possible to ensure that the desired orientation was maintained during the microshear bond strength test. Pulling force was applied at the crosshead speed of 1.0 mm/min until failure occurred (Figure 2). Data were statistically analyzed using one-way ANOVA and post-hoc Schèffe test at the 95% level of confidence.
The mean values and the standard deviations of microshear bond strength are shown in Table 2. The control group (group 4, Con) showed the highest mean bond strength followed by Claren (group 2, Cla), Crest (group 3, Cre) and Opalescence gel (group 1, Opal). Although the microshear bond strength values were numerically different in the previous order, one-way ANOVA and post-hoc Schèffe test showed no variations between the test groups and no statistically significant differences in microshear bond strength values between the groups (p > 0.05).
For bleached enamel surfaces, there was a tendency of reduction of the microshear bond strength of either bleaching agents compared to the unbleached ones. However, this study indicated no statistically significant differences in bond strength values between the groups. This result is consistent with the study of Josey et al.7), who examined the shear bond strength of composite resin luting cement to etched bleached enamel. The study showed no significant difference between the bleached and unbleached enamels and indicated that either appeared to be clinically acceptable.
On the other hand, there were studies with the opposite results: reductions in bond strength after enamel bleaching with 10% carbamide peroxide6,8-10). One reason for the reduced bond strength was said to be the result of the changes in enamel structures during the bleaching procedure. Ben-Amar et al.11) cited in their study that these changes in enamel structures resulted from loss of mineral content or increased porosity manifested by an "over-etched" appearance with loss of prismatic form. Josey et al.7) addressed that the characteristics of the etched enamel following bleaching appear to be altered and may affect the bond of composite resin to bleached enamel. Other studies demonstrated surface alterations in enamel with the bleaching agents,12,13) and loss of calcium and changes in calcium/phosphate ratio after applying carbamide peroxide on enamel12-14). However, studies on the effect of bleaching gels on enamel surfaces with 10% carbamide peroxide addressed that bleaching gels caused only minimal effect on the surface morphology and enamel microhardness4,12,14-16). Lai et al.17) reported in their study that 10% carbamide peroxide bleaching produced only slight enamel surface alterations and this etching effect was likely to be pH-dependent. They also cited that it could be expected that the demineralization effect of bleaching agents was relatively mild and any surface and subsurface alterations would probably have been masked by the more aggressive phosphoric acid etching.
Another reason for the reduced bond strength of bleached enamel is the residual hydrogen peroxide after bleaching. Titely et al.9) stated in their study that the reduction in bond strength might be related to the presence of residual hydrogen peroxide which interfered with resin attachment and inhibited resin polymerization at or near the enamel surface and they showed that the immersion of hydrogen peroxide treated teeth in water could leach out the hydrogen peroxide and restore bond strength values. Since dental adhesives polymerize by a free radical polymerization mechanism that involves the generation of free radicals through light-activated redox initiators,18) the hydrogen peroxide may break down to release oxygen that is trapped within the adhesive during light-activation. The released oxygen from the bleached enamel probably results in incomplete polymerization of the adhesive in these regions10,19). However, Adibfar et al.20) stated that water immersion can effectively remove all of the hydrogen peroxide from the bovine enamel in a matter of minutes. In that study, the dentin was removed to eliminate the possible reservoir for the hydrogen peroxide which is transported through the full thickness of the enamel. In this study, the blocks were stored in the artificial saliva with the exception of the bleaching time, so it can be said that there was sufficient time for the residual hydrogen peroxide to be removed from the test specimen. There were other methods to remove the residual hydrogen peroxide. Lai et al.17) reported that compromised bonding to acid-etched bleached enamel was reversed with antioxidant (e.g. sodium ascorbate) and that altered subsurface enamel organic matrix by oxidizing effect of hydrogen peroxide could be reversible changes in redox potential of the organic components.
One of the problems related with complicated adhesive systems in bonding composite resin to enamel is the time required for the various steps and the associated technique sensitivity. To overcome these shortcomings, systems such as self-etching and priming adhesive materials are used21). Therefore, self-etching adhesive (Adper Prompt L-Pop) was used in this study. In the study of Pashley et al.22) on the etching effects of self-etching adhesives, Prompt L-Pop (3M ESPE, St Paul, USA) produced an etching effect that approached the total-etch control group.
In this study, composite resin adhesion to bleached enamel was assessed by the microshear bond strength. Various mechanical test methods, such as shear and tensile strength tests, have been used to evaluate the bond strength. The tensile test is very critical. If not carefully conducted, the specimen undergoes torque stress, which reduces the bond strength value. The shear tests are used more often because the test values are higher than those obtained by the tensile test and the shear stress is considered to be more representative of the clinical situation23). Moreover, by virtue of its overall simplicity, such as the ease of specimen preparation, simple test protocol, and the ability to rank different products according to bond strength values, several variations of shear bond testing have been widely applied. However, the tendency of failure in the dentinal substrate at loads far less than the shear strength of the dentin due to improved bond strength by the new adhesives, would limit the ability of the test to discern real differences among various adhesive systems24). Pashley et al.25) cited this particular issue as a limitation of using conventional shear tests and a reason to consider the microshear test as a reasonable alternative. Tantbirojn et al.26) addressed concern that shear test may not be able to estimate bond strength if future generations of bonding systems get better. If the dentin pull-out was observed in the failure surface, the calculated nominal bond strength was no longer based on the cross-sectional area. Thus, they concluded that the bond test could not discriminate the effect of such new bonding agents. However, when using microshear bond strength test method, there is less risk of previously mentioned shortcomings. Furthermore only small tooth segments are needed and this permits many grouping on the same substrate, as well as promoting the conservation of extracted teeth24).
In this study, the microshear bond strength values were variable even in the same group. The irregularities of the unground enamel surface are suspected to be the reason. Because of these irregularities of the enamel surface, the actual bonded surface conditions of composite resin cylinder could be different each other and result in the differences of the microshear bond strength. To eliminate these possibilities, the composite resin bonding to the flattened enamel subsurface should be taken into consideration in further studies. When the irregularities of enamel surfaces could be excluded, the standard deviation of bond strength values would be reduced.
In this in vitro study, microshear bond strength of composite resin to enamel bleached with commercially available bleaching agents was evaluated. Twelve extracted central incisors were cut into squares of 2×2 mm in size and most central 4 segments were obtained from each tooth. One segment from each tooth was chosen and embedded in acrylic resin to obtain 4 groups. Group 1 was bleached with Opalescence tooth whitening gel with 10 % carbamide peroxide, group 2 with Claren dental whitening solution, group 3 with Crest whitestrips and group 4 was used as a control. After 14 days of bleaching, composite resin cylinder was bonded to the bleached enamel surfaces with the help of Tygon tube using the self-etching adhesive. The universal testing machine was used to conduct the microshear bond strength test. The bond strength was highest in the control group and lowest in the Opalescence gel group, but there was no statistically significant difference (p > 0.05). Therefore, it can be stated that bleaching with commercially available whitening strips and the bleaching gel with 10 % carbamide peroxide did not influence the microshear bond strength of composite resin to enamel.
Figures and Tables
Figure 1
Tooth embedded in resin specimen was cut into squares of 2×2 mm in size and the most central 4 segments were chosen.
References
1. Cavalli V, Reis AF, Giannini M, Ambrosano GMB. The effect of elapsed time following bleaching on enamel bond strength of resin composite. Oper Dent. 2001. 26(6):597–602.
2. Rodrigues JA, Basting RT, Serra MC, Rodrigues AL Jr. Effects of 10% carbamide peroxide bleaching materials on enamel microhardness. Am J Dent. 2001. 14:67–71.
3. Gerlach RW, Xiaojie Z. Vital bleaching with whitening strips: summary of clinical research on effectiveness and tolerability. J Contemp Dent Pract. 2001. 2(3):1–16.

4. Hegedüs C, Bistey T, Flóra-Nagy E, Keszthelyi G, Jenei A. An atomic force microscopy study on the effect of bleaching agents on enamel surface. J Dent. 1999. 27(7):509–515.

5. Haywood VB, Heymann HO. Nightguard vital bleaching. Quintessence Int. 1989. 20(3):173–176.
6. Sung EC, Chan SM, Mito R, Caputo AA. Effect of carbamide peroxide bleaching on the shear bond strength of composite to dental bonding agent enhanced enamel. J Prosthet Dent. 1999. 82(5):595–599.

7. Josey AL, Meyers IA, Romaniuk K, Symons AL. The effect of a vital bleaching technique on enamel surface morphology and the bonding of composite resin to enamel. J Oral Rehabil. 1996. 23(4):244–250.

8. Garcia-Godoy F, Dodge WW, Donohue M, O'Quinn JA. Composite resin bond strength after enamel bleaching. Oper Dent. 1993. 18(4):144–147.
9. Titley KC, Torneck CD, Ruse ND, Krmec D. Adhesion of a resin composite to bleached and unbleached human enamel. J Endod. 1993. 19(3):112–115.

10. Dishman MV, Covey DA, Baughan LW. The effects of peroxide bleaching on composite to enamel bond strength. Dent Mater. 1994. 10(1):33–36.

11. Ben-Amar A, Liberman R, Gorfil C, Bernstein Y. Effect of mouthguard bleaching on enamel surface. Am J Dent. 1995. 8(1):29–32.
12. Akal N, Over H, Olmez A, Bodur H. Effects of carbamide peroxide containing bleaching agents on the morphology and subsurface hardness of enamel. J Clin Pediatr Dent. 2001. 25(4):293–296.
13. Basting RT, Rodrigues AL Jr, Serra MC. The effect of 10% carbamide peroxide bleaching material on microhardness of sound and demineralized enamel and dentin in situ. Oper Dent. 2001. 26(6):531–539.
14. Cimilli H, Pameijer CH. Effect of carbamide peroxide bleaching agents on the physical properties and chemical composition of enamel. Am J Dent. 2001. 14(2):63–66.
15. Potocnik I, Kosec L, Gaspersic D. Effect of 10% carbamide peroxide bleaching gel on enamel microhardness, microstructure, and mineral content. J Endod. 2000. 26(4):203–206.

16. Leonard RH, Eagle JC, Garland GE, Matthews KP, Rudd AL, Phillips C. Nightguard vital bleaching and its effect on enamel surface morphology. J Esthet Restor Dent. 2001. 13(2):132–139.

17. Lai SC, Tay FR, Cheung GS, Mak YF, Carvalho RM, Wei SHY, Toledano M, Osorio R, Pashley DH. Reversal of compromised bonding in bleached enamel. J Dent Res. 2002. 81(7):477–481.

18. Monroe BM, Weiner SA, Hammond GS. Mechanisms of photochemical reactions in solution. Photoreduction of camphoroquinone. J Am Chem Soc. 1968. 90(7):1913–1914.

19. Torneck CD, Titley KC, Smith DC, Adibfar A. The influence of time of hydrogen peroxide exposure on the adhesion of composite resin to bleached bovine enamel. J Endod. 1990. 16(3):123–128.

20. Adibfar A, Steele A, Torneck CD, Titley KC, Ruse ND. Leaching of hydrogen peroxide from bleached bovine enamel. J Endod. 1992. 18(10):488–491.

21. Shimada Y, Senawongse P, Harnirattisai C, Burrow MF, Nakaoki Y, Tagami J. Bond strength of two adhesive systems to primary and permanent enamel. Oper Dent. 2002. 27(4):403–409.
22. Pashley DH, Tay FR. Aggressiveness of contemporary self-etching adhesives Part II: etching effects on unground enamel. Dent Mater. 2001. 17(5):430–444.

23. Cardoso PE, Braga RR, Carrilho MRO. Evaluation of micro-tensile, shear and tensile tests determining the bond strength of three adhesive systems. Dent Mater. 1998. 14(6):394–398.

24. McDonough WG, Antonucci JM, He J, Shimada Y, Chiang MYM, Schumacher GE, Schultheisz CR. A microshear test to measure bond strengths of dentin-polymer interfaces. Biomaterials. 2002. 23(17):3603–3608.

25. Pashley DH, Carvalho RM, Sano H, Nakajima M, Yoshiyama M, Shono Y, Fernandes CA, Tay F. The microtensile bond test: a review. J Adhes Dent. 1999. 1(4):299–309.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download