Abstract
The purpose of this study was to evaluate the effect of EDTA irrigant according to application time and temperature.
31 human mature extracted teeth with a single canal were sectioned with microtome in 3mm thickness and gained 62 samples of root canals. They were distributed randomly into 6 groups of 10 specimens each and control group of 2 specimens. Each specimen was prepared with GT rotary file (Dentsply, Maillefer Co., Swiss) and irrigated with 3 ml sodium hypochlorite every minute. Then smear layer was removed with EDTA solution (PULPDENT®, PULPDENT Co., USA.) except two control specimens. Specimens of each group were irrigated with 17% EDTA.
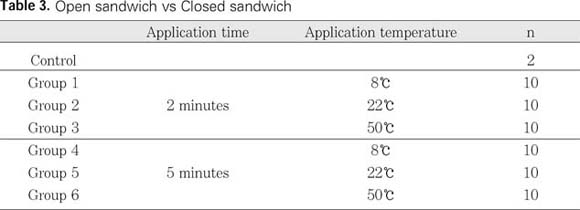
The time and temperature of application were as follows:
All specimens were split longitudinally and prepared for examination by scanning electron microscopy. A set of reference micrographs was used to award a debris score as follows: 0 = no smear layer, all tubules clean and open; 1 = no superficial smear layer, tubule openings visible, but some contain debris plug or soft tissue remnants; 2 = moderate smear layer, some tubules open and others closed; 3 = heavy smear layer, most/all tubule openings obscured. Results were evaluated with Kruskal-Wallis test to determine whether there was statistically significant difference among six groups. Pairs of groups were analyzed using the Student-Newman-Keuls Method and Mann-Whitney test.
The results were as follows:
1. Control specimens showed heavy smear layer at the canal walls.
2. Among the groups applied with EDTA for 2 minutes, group 1 showed the heaviest smear layer, and there was statistically significant difference between group 1 and the other groups(p<0.05).
3. Among the groups applied with EDTA for 5 minutes, group 4 and group 6 showed smear layer, but there was no significant difference between them.
4. Among the groups applied with EDTA for the same temperature, group 1 showed heavier smear layer than group 4, and there was statistically significant difference(p<0.05).
5. Among the groups applied with EDTA for the same temperature, group 2 showed heavier smear layer than group 5 and group 3 showed heavier smear layer than group 6. But there was no statistically significant difference among them.
From the results above, it could be concluded, EDTA solution is effective in removing of smear layer when it is applied for 5 minutes. If EDTA is applied for 2 minutes, it should be applied above room temperature.
Figures and Tables
References
1. McComb D, Smith DC. A preliminary scanning electron microscopic study of root canals after endodontic procedures. J Endod. 1975. 1:238–242.

2. Vojinovic O, Nyborgh H, Brannstom M. Acid treatment of cavities under resin fillings: Bacterial growth in dentinal tubules and pulpal reactions. J Dent Res. 1973. 52:1189–1193.

3. Diamond A, Carrel R. The smear layer: a review of restorative process. J Pedod. 1984. 8:219–226.
4. Baker NA, Eleazer PD, Averbach RE, Seltzer S. Scanning electron microscopic study of the efficacy of various irrigaing solutions. J Endod. 1975. 1:127–135.

5. Yamada RS, Armas A, Goldman M, Lin PS. A Scanning electron microscopic comparison of a high volume final flush with several irrigating solutions: Part III. J Endod. 1983. 9:137–142.

6. Pashley DH, Livingstone MJ. Effect of molecular size on permeability coefficients in human dentine. Arch Oral Biol. 1978. 23:391–395.

7. Uitto VJ, Haapasalo M, Laakso T, Salo T. Degradation of basement membrane(Type IV) collagen by proteases from some anaerobic microorganisms. Oral Microbiol Immunol. 1973. 3:97–102.

8. Pitt Ford TR, Roberts GJ. Tissue response to glass ionomer retrograde root fillings. Int Endod J. 1990. 23:233–238.

9. Ingle JI. Endodontics. 3rd edn. Philadelphia, PA, USA: Lea and Febiger;178–180.
10. Rubin LM, Skobe F, Krakow A, Gron P. The effect of instrumentation and flushing of freshly extracted teeth in endodontic therapy: a scanning electron microscope study. J Endod. 1979. 5:328–335.

11. Moorer WR, Wesselink PR. Factors promoting the tissue dissolving capability of sodium hypochlorite. Int Endod J. 1982. 15:187–196.

13. Patterson S. In vivo and in vitro studies of the effect of the disodium salt of EDTA on human dentin and its endodontic implications. Oral Surg. 1963. 16:83–103.

14. Nikiforuk G, Sreebny L. Demineralization of hard tissues by organic chelating agents. Science. 1951. 114:560.

15. Gambarini G. Shaping and cleaning the root canal system: a scanning electron microscopic evaluation of a new instrumentation and irrigation technique. J Endod. 1999. 25:800–803.

16. Brannstrom M, Nordenvall KJ, Grantz PO. The effect of EDTA-containing surface-active solutions on the morphology of prepared dentin: an in vivo study. J Dent Res. 1980. 59:1127–1131.

17. Cameron JA. The use of ultrasound for the removal of the smear layer. The effect of sodium hypochlorite concentrations; SEM study. Aust Dent J. 1988. 33:193–200.

18. Eick JD, Wilko RA, Anderson CH, Sorensen SE. Scanning electron microscopy of cut tooth surfaces and identification of debris by use of the electron microprobe. J Dent Res. 1970. 49:1359–1368.

19. Pashley DH, Tao L, Boyd L, King GE, Horner JA. Scanning electron microscopy of the substructure of smear layers in human dentin. Arch Oral Biol. 1988. 33:265–270.

20. Mader CL, Baumgartner JC, Peters DD. Scanning electron microscopy investigation of the smeared layer on root canal walls. J Endod. 1984. 10:477–483.

21. Cengiz T, Aktener BO, Piskin B. The effect of dentinal tubule orientation on the removal of smear layer by root canal irrigants. A scanning electron microscopic study. Int Endod J. 1990. 23:163–171.

22. Perez F, Calas P, De Falguerolles A, Maurette A. Migration of a streptococcus sanguis strain through root dental tubules. J Endod. 1993. 19:297–301.

23. Kennedy WA, Walker WA, Gough RW. Smear layer removal effects on apical leakage. J Endod. 1986. 12:21–27.

24. Wayman BE, Kopp WM, Pinero GJ, Lazzari EP. Citric and lactic acids as root canal irrigants in vitro. J Endod. 1979. 5:258–265.
25. Bitter NC. A 25% tannic acid solution as a root canal irrigant cleanser: a scanning electron microscope study. Oral Surg. 1989. 67:333–337.

26. McComb D, Smith DC, Beagrie GS. The results of in vitro endodontic chemomechanical instrumentation-a scanning electron microscope study. J Br Endod Soc. 1976. 9:11–18.

27. Takeda FH, Harashima T, Kimura Y, Matsumoto K. A comparative study of the removal of smear layer by three endodontic irrigants and two types of laser. Int Endod J. 1999. 32:32–39.

28. Fehr FR, Nygaard-Ostby . Effect of EDTA and sulphuric acid on root canal dentine. Oral Surg. 1963. 16:199–205.
29. Fraser JG. Chelating agents: Their softening effect on root canal dentin. Oral Surg. 1974. 37:803–811.

30. Biesterfeld RC, Taintor JF. A comparison of periapical seal of root canals with RC-Prep or Salvizol. Oral Surg. 1980. 49:532–537.




 PDF
PDF ePub
ePub Citation
Citation Print
Print















 XML Download
XML Download