Abstract
Bacterial lipopolysaccharide (LPS) plays a major role in stimulating the synthesis and release of the principal osteoclast-activating cytokines, namely, interleukin 1 and tumor necrosis factor-α from immune cells. Although monocytes/macrophages are the main producers of these cytokines, recent evidence has indicated that polymorphonuclear leukocytes (PMN) have the ability to release IL-1 and TNF-α. Calcium hydroxide has been shown to be an effective medicament in root canal infections, reducing the microbial titre within the canal. It has been proposed that the therapeutic effect of Ca(OH)2 may also be the result of direct inactivation of LPS. The purpose of this study was to investigate whether treatment of Porphyromonas endodontalis LPS with calcium hydroxide alters its biological action as measured by human PMN secretion of IL-1 and TNF-α, and it was compared with Escherichia coli LPS.
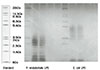
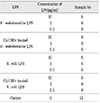
P. endodontalis ATCC 35406 was cultured in anaerobic condition, and LPS was extracted using the hot-phenol water extraction method and purified. Purchased E. coli LPS was also purified. 100 µg/ml of each LPS in pyrogen free water were incubated with 25mg/ml Ca(OH)2 at 37℃ for 7 days. The supernatants were subjected to ultrafiltration, and the isolates were lyophilized and weighed. PMNs were obtained from peripheral blood by centrifugation layered over Lymphoprep. The cells were resuspended (4×106 cells/ml) in RPMI 1640 followed by treatment with various concentrations of LPS (0, 0.1, 1, 10µg/ml) for 24 hours at 37℃ in 5% CO2 incubator. The supernatants of cells were collected and the levels of IL-1α, IL-1β and TNF-α were measured by enzyme-linked immunosorbent assay.
The results were as follows;
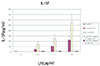
1. The levels of IL-1α, IL-1β, TNF-α from PMN treated with each LPS were significantly higher than those released from unstimulated PMN of the control group (p<0.05).
2. The levels of all three cytokines released from PMN stimulated with each calcium hydroxide treated LPS were significantly lower than those released from PMN stimulated with each untreated LPS (p<0.05), while they were not significantly different from those released from unstimulated PMN of the control group (p>0.05).
3. The levels of secretion for all three cytokines were affected in a dose-dependent manner in PMN stimulated with each LPS (p<0.05), but not in PMN stimulated with each calcium hydroxide treated LPS (p>0.05).
4. The levels of all three cytokines released from PMN stimulated with P. endodontalis LPS were significantly lower than those released from PMN stimulated with E. coli LPS (p<0.05).
Figures and Tables
References
1. Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965. 20:340–349.

2. Bystrom A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981. 89:321–328.

3. Bystrom A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985. 18:35–40.

4. Siqueira JF Jr, Machado AG, Silveira RM, Lopes HP, Uzeda M. Evaluation of the effectiveness of sodium hypochlorite used with three irrigation methods in the elimination of Enterococcus faecalis from the root canal. Int Endod J. 1997. 30:279–282.

5. Bystrom A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985. 1:170–175.

6. Hasselgren G, Olsson B, Cvek M. Effects of calcium hydroxide and sodium hypochlorite in the dissolution of necrotic porcine muscle tissue. J Endod. 1988. 14:125–127.

7. Andersen M, Lund A, Andreasen JO, Andreasen FM. In vitro solubility of human pulp tissue in calcium hydroxide and sodium hypochlorite. Endod Dent Traumatol. 1992. 8:104–108.

8. Tronstad L. Root resorption - etiology, terminology and clinical manifestations. Endod Dent Traumatol. 1988. 4:241–252.

10. Heithersay GS. Calcium hydroxide in the treatment of pulpless teeth with associated pathology. J Br Endod Soc. 1975. 8:74–93.

11. Fava LR, Saunders WP. Calcium hydroxide pastes: classification and clinical indications. Int Endod J. 1999. 32(4):257–282.

12. Siqueira JF Jr, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. 1999. 32:361–369.

13. Safavi KE, Nichols FC. Effect of calcium hydroxide on bacterial lipopolysaccharide. J Endod. 1993. 19:76–78.

14. Mattsby-Baltzer I, Lindgren K, Lindholm B, Edebo L. Endotoxin shedding by enterobacteria: free and cell-bound endotoxin differ in Limulus activity. Infect Immun. 1991. 59:689–695.

15. Bystrom A, Happonen RP, Sjogren U, Sundqvist G. Healing of periapical lesions of pulpless teeth after endodontic treatment with controlled asepsis. Endod Dent Traumatol. 1987. 3(2):58–63.

16. Ørstavik D, Kerekes K, Molven O. Effects of extensive apical reaming and calcium hydroxide dressing on bacterial infection during treatment of apical periodontitis. Int Endod J. 1991. 24:1–7.

17. Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zahringer U, Seydel U, Di Padova F, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994. 8(2):217–225.

18. Morrison DC, Ryan JL. Endotoxin and disease mechanisms. Ann Rev Med. 1987. 38:417–432.
19. Niwa M, Milner KC, Ribi E, Rudbach JA. Alteration of physical, chemical and biological properties of endotoxin by treatment with mild alkali. J Bacteriol. 1969. 97:1069–1077.

20. Wang CY, Stashenko P. Kinetics of bone-resorbing activity in developing periapical lesions. J Dent Res. 1991. 70:1362–1366.

21. Le J, Vilcek J. Tumor necrosis factor and interleukin 1 : Cytokines with multiple overlapping biological activities. Lab Invest. 1987. 56:234–248.
22. Nguyen L, Dewhirst FE, Hauschka PV, Stashenko P. Interleukin 1βstimulates bone resorption and inhibits bone formation in vivo. Lymphokine Cytokine Res. 1990. 10:15–21.
23. Lloyd AR, Oppenheim JJ. Poly's lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol Today. 1992. 13:169–172.

24. Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995. 16(1):21–26.

25. Buck RA, Cai J, Eleazer PD, Staat RH, Hurst HE. Detoxification of endotoxin by endodontic irrigants and calcium hydroxide. J Endod. 2001. 27(5):325–327.

26. Safavi KE, Nichols FC. Alteration of biological properties of bacterial lipopolysaccharide by calcium hydroxide treatment. J Endod. 1994. 20:127–129.

27. Barthel CR, Levin LG, Reisner HM, Trope M. TNF-α release in monocytes after exposure to calcium hydroxide treated Escherichia coli LPS. Int Endod J. 1997. 30:155–159.

28. Sundqvist G. Prevalence of black-pigmented bacteroides species in root canal infections. J Endod. 1989. 15:13–19.

29. Haapasalo M, Ranta H, Ranta K, Shah H. Black-pigmented bacteroides spp. in human apical periodontitis. Infect Immun. 1986. 53:149–153.

30. Baumgartner JC, Falkler WA. Bacteria in the apical 5mm of infected root canals. J Endod. 1991. 17:380–383.
31. Hashioka K, Yamasaki M, Nakane A, Horiba N, Nakamura H. The relationship between clinical symptoms and anaerobic bacteria from infected root canals. J Endod. 1992. 18:558–561.

32. Ko HJ, Lim SS. Effects of Porphyromonas endodontalis lipopolysaccharide on IL-1β, TNF-α and IL-1ra production by human polymorphonuclear leukocytes. 2001. Korea: Dept. of Conservative Dentistry, Seoul National University;PhD thesis.
33. Westphal O, Jann K. Bacterial lipopolysaccharides : extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965. 5:83–91.
34. Koga T, Nishihara T, Fujiwara T, Nisizawa T, Okahashi N, Noguchi T, Hamada S. Biochemical and immunobiological properties of lipopolysaccharide from Bacteroides gingivalis and comparison with LPS from Escheria coli. Infect Immun. 1985. 47(3):638–647.

35. Pitts DL, Williams BL, Morton TH Jr. Investigation of the role of endotoxin in periapical inflammation. J Endod. 1982. 8(1):10–18.

36. Sjogren U, Figdor D, Spangberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J. 1991. 24(3):119–125.

37. Stuart KG, Miller CH, Brown CE Jr, Newton CW. The comparative antimicrobial effect of calcium hydroxide. Oral Surg Oral Med Oral Pathol. 1991. 72(1):101–104.

38. Lim SS. Clinical Endodontics. 1994. Korea: Uichihaksa;123.
39. Martin DM, Crabb HS. Calcium hydroxide in root canal therapy. A review. Br Dent J. 1977. 142(9):277–283.

40. Munford RS, Hall CL. Detoxification of bacterial lipopolysaccharides(endotoxins) by a human neutrophil enzyme. Science. 1986. 234:203–205.

41. Nilsen R, Johannessen AC, Skaug N, Matre R. In situ characterization of mononuclear cells in human dental periapical inflammatory lesions using monoclonal antibodies. Oral Surg Oral Med Oral Pathol. 1984. 58:160–165.

42. Stern MH, Dreizen S, Mackler BF, Levy BM. Isolation and characterization of inflammatory cells from the human periapical granuloma. J Dent Res. 1982. 61:1408–1412.

43. Yu SM, Stashenko P. Identification of inflammatory cells in developing rat periapical lesions. J Endod. 1987. 13:535–540.

44. Stashenko P. Role of immune cytokines in the pathogenesis of periapical lesions. Endod Dent Traumatol. 1990. 6:89–95.
45. Ko HJ, Chung KH, Lim SS. Tissue levels of IL-1α, IL-1βand TNF-αin pulpal and periapical pathosis. J Korean Acad Conserv Dent. 1998. 23(1):316–327.
46. Byun HY, Lim SS, Park DS. Levels of TNF-α,-β, IL-β, TGF-β1 and their relationship with the presence of specific black pigmented bacteria in periapical and pulpal diseases. J Korean Acad Conserv Dent. 1999. 24(1):1–12.
47. Gong HG, Park DS, Lim SS. Levels of IL-1 and TNF-α in odontogenic cyst & cystic fluid. J Korean Acad Conserv Dent. 1999. 24(1):49–54.
48. Ogura N, Shibata Y, Kamino Y, Matsuda U, Hayakawa M, Oikawa T, Takiguchi H, Izumi H, Abiko Y. Stimulation of interleukin-6 production of periodontal ligament cells by Porphyromonas endodontalis lipopolysaccharide. Biochem Med Metab Biol. 1994. 53(2):130–136.

49. Hosoya S, Matsushima K, Ohbayashi E, Yamazaki M, Shibata Y, Abiko Y. Stimulation of interleukin-1beta-independent interleukin-6 production in human dental pulp cells by lipopolysaccharide. Biochem Mol Med. 1996. 59(2):138–143.

50. Hosoya S, Matsushima K. Stimulation of interleukin-1 beta production of human dental pulp cells by Porphyromonas endodontalis lipopolysaccharide. J Endod. 1997. 23(1):39–42.

51. Hofstad T, Skaug N, Sveen K. Stimulation of B lymphocytes by LPS from anaerobic bacteria. Clin Infect Dis. 1993. 16:Suppl 4. S200–S202.
52. Yoshimura A, Hara Y, Kaneko T, Kato I. Secretion of IL-1β, TNF-α, IL-8 and IL-1ra by human polymorphonuclear leukocytes in response to lipopolysaccharides from periodontopathic bacteria. J Periodont Res. 1997. 32:279–286.

53. Matsushita K, Tajima T, Tomita K, Takada H, Nagaoka S, Torii M. Inflammatory cytokine production and specific antibody responses to lipopolysaccharide from endodontopathic black-pigmented bacteria in patients with multilesional periapical periodontitis. J Endod. 1999. 25:795–799.

54. Hanazawa S, Sagiya T, Kitami H, Ohta K, Nishikawa H, Kitano S. Monoclonal antibody against Porphyromonas (Bacteroides) endodontalis lipopolysaccharide and application of the antibody for direct identification of the species. J Clin Microbiol. 1991. 29(11):2550–2553.

55. Eidhin DN, Mouton C. A rapid method for preparation of rough and smooth lipopolysaccharide from Bacteroides, Porphyromonas and Prevotella. FEMS Microbiol Lett. 1993. 110(2):133–138.

56. Kim JH, Kim MK, Yoon SH. Effects of Porphyromonas endodontalis lipopolysaccharide on membrane permeability of fibroblast. J Korean Acad Conserv Dent. 1999. 24(3):437–446.
57. Schein B, Schilder J. Endotoxin content in endodontically involved teeth. J Endod. 1975. 1:19–21.

58. Horiba N, Maekawa Y, Abe Y, Ito M, Matsumoto T, Nakamura H. Correlations between endotoxin and clinical symptoms or radiolucent areas in infected root canals. Oral Surg Oral Med Oral Pathol. 1991. 71(4):492–495.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download