Abstract
One fifth dilution of formocresol is usually used for pulpotomy of the primary teeth and emergency pulpotomy of the permanent teeth. However, the use of formaldehyde has been subjected to criticism because it may be absorbed into the blood stream and become distributed systemically, it may also alter the pulp tissue rendering it immunologically active, and have carcinogenic potential. Recently Depulpin®(VoCo., Germany) gains popularity as a devitalizing agent during root canal therapy in spite of high concentration of 49% paraformaldehyde because it facilitate devitalization of pulp and make root canal therapy easier. But there have been not enough publications about the reaction of pulp and periapical tissue caused by Depulpin.
This study was performed to evaluate the histological changes in pulp and periapical tissue of rats after pulpotomy using formocresol and Depulpin and to elucidate the toxic effects of these agents. Thirty six Sprague-Dawley rats were anesthetized by intraperitoneal injection of ketamine. Maxillary first molar teeth were used for pulpotomy with formocresol and Depulpin. Rats were sacrificed after 2 days, 4 days, 1 week, 2 weeks, 3 weeks and 4 weeks respectively. Specimens were histologically observed by light microscope changes in pulp and periapical tissue. The obtained results were as follows.
1. Formocresol group
A zone of fixed tissue, in which odontoblasts could clearly be defined, was present directly underneath the pulpotomy dressing in almost all teeth of this group. This was followed by an area of necrotic tissue which resembled dried out fibrous tissue with no cellular detail except some pyknotic nuclei. In the specimens of after 2 days, 4 days, 1 week, 2 weeks in which vital tissue was present, it was separated from the fibrous area by a zone of inflammation. In the specimens of after 3 weeks and after 4 weeks, inflammatory infiltrate was in the periodontal ligament adjacent to the apical foramina of the teeth.
2. Depulpin® group
The area of necrotic tissue which had no cells and fibers, was present adjacent to the dressing. This was followed by dried out fibrous tissue with no cellular details except some pyknotic nuclei. A short stump of vital pulp with odontoblasts was present at the end of the canal after 2 days. Inflammatory infiltrate was in the periodontal ligament after 4 days and after 1week. Severe root resorption and necrosis of periapical tissue opposite the root resorption site were defined after 2 weeks and after 3 weeks. Periapical lesion which consist of necrotic tissue surrounded by a fibrous connective wall, was found after 4 weeks.
The results indicated that Depulpin can cause more adverse reaction to the dental pulp and periapical tissue than formocresol, and further studies are needed for its clinical use with safety.
Figures and Tables
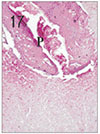
Fig. 4
Apical pulp tissue and periapical tissue after 2 days of application of Depulpin®. H & E stain, ×100.
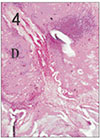
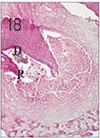
Fig. 5
Apical pulp tissue and periapical tissue after 4 days of application of formocresol. No histologic change was observed in periodontal ligament. H & E stain, ×100.
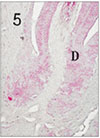
Fig. 6
Pulp tissue after 4 days of application of formocresol. Dilatation of vessel was observed. H & E stain, ×400.
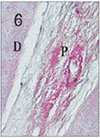
Fig. 7
Pulp tissue and periapical tissue after 4 days of application of Depulpin®. Periodontal ligament opposite the apical foramen was altered. H & E stain, ×100.
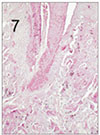
Fig. 8
Pulp tissue after 1 week of application of formocresol. Vital pulp tissue was observed in the apical area. H & E stain, ×40.
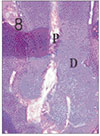
Fig. 9
Pulp tissue after 1 week of application of formocresol. No histologic change was observed in periodontal ligament. H & E stain, ×200.
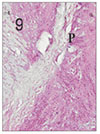
Fig. 10
Pulp tissue and periapical tissue after 1 week of application of Depulpin®. H & E stain, ×100.
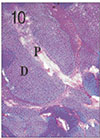
Fig. 11
Pulp tissue and periapical tissue after 2 weeks of application of formocresol. H & E stain, ×40.
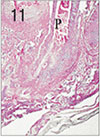
Fig. 12
Apical pulp tissue and periapical tissue after 2 weeks of application of Depulpin®. Complete necrosis of pulp and resorption of alveolar socket bone was observed. H & E stain, ×100.
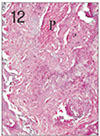
Fig. 13
Apical pulp tissue and periapical tissue after 3 weeks of application of formocresol. Inflammatory infiltrates were observed in periodontal ligament opposite the apical foramen. H & E stain, ×200.
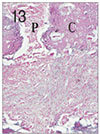
Fig. 14
Pulp tissue and periapical tissue after 3 weeks of application of Depulpin®. Complete pulpal necrosis,
root resorption and bone resorption were observed. H & E stain, ×40.
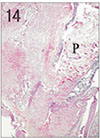
Fig. 15
Pulp tissue and periapical tissue after 3 weeks of application of Depulpin®. Complete pulpal necrosis, root resorption and bone resorption were observed. H & E stain, ×100.
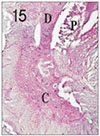
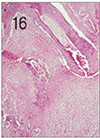
Fig. 16
Pulp tissue and periapical tissue after 4 weeks of application of formocresol. Coronal pulp was fixed. Severe inflammatory infiltrations in the periapical area were shown. H & E stain, ×40.
References
1. Cotes O, Boj JR, Canalda C, Carreras M. Pulpal tissue reaction to formocresol vs ferric sulfate in pulpotomized rat teeth. J Clin Pediatr Dent. 1997. 21:247–254.
2. Waterhouse PJ. Formocresol and alternative primary molar pulpotomy medicaments : A review. Endod Dent Traumatol. 1995. 11:157–162.

3. Tagger E, Tagger M. Pulpal and periapical reaction to glutaraldehyde and paraformaldehyde pulpotomy dressing in monkeys. J Endod. 1984. 10:364–371.

4. Wesley DJ, Marshall FS, Rosen S. The quantitation of formocresol as a root medicament. Oral Surg. 1970. 29:603–612.
5. Morawa AP, Straffon LH, Han SS, Corpron RE. Clinical evaluation of pulpotomies using dilute formocresol. ASDC J Dent Child. 1975. 42:360–363.
6. Seltzer S, Bender IB. The dental pulp. Biologic consideration in dental procedures. 1990. 3rd ed. ST. Louis: J.B. Lippincott Co;215–237.
7. Straffon LH, Han SS. Effect of varying concentrations of formocresol on RNA synthesis of connective tissue in sponge implants. Oral Surg. 1970. 29:915–925.

8. Langer K, Dowden WE, Tronstad L, Langeland LK. Human pulp changes of iatrogenic origin. Oral Surg. 1971. 32:943–949.

9. Myers DR, Shoaf HK, Dirksen TR, Pashley DH, Whitford GM, Reynold KE. Distribution of 14Cformaldehyde after pulpotomy. J Am Dent Assoc. 1977. 94:698–700.
10. Pruhs RJ, Olen GA, Sharma PS. Relationship between formocresol pulpotomies on primary teeth and enamel defects on their permanent successors. J Am Dent Assoc. 1977. 94:698–700.

11. Swernberg JA, Krens WD, Mitchell RJ, Gralla EJ, Pavcov KL. Introduction of squamous cell carcinoma of the rat nasal cavity by inhalation exposure to formaldehyde vapour. Cancer Res. 1980. 40:3398–3402.
12. Korean Academy of Pediatric Dentistry. Clinical Study on Children & Adolescence. 1999. 275.
13. Hill SD, Berry CW, Seale NS, Kaga M. Comparison of antimicrobial and cytotoxic effects of glutaraldehyde and formocresol. Oral Surg Oral Med Oral Pathol. 1991. 71:89–95.

14. Pashley DR, Myers DR, Pashley DH, Whitford GM. Systemic distribution of 14C-formaldehyde from formocresol treated pulpotomy sites. J Dent Res. 1980. 59:603–608.
15. Wilkins FJ, Macleod HD. Formaldehyde introduced DNA-protein crosslinks in Escerichia coli. Mutat Res. 1976. 36:11–16.
16. Friedberg BH, Gartner LP. Embriotoxicity of formocresol on developing chick embryos. J Endod. 1990. 16:434–437.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download