Abstract
Background:
A piroxicam patch has been widely used to treat musculoskeletal pain. The aim of this study was to assess the pharmacokinetic (PK) characteristics and skin irritation of Murupe® patch (piroxicam 96 mg) compared with Trast® patch (piroxicam 96 mg) in healthy volunteers.
Methods:
A randomized, open-label, 2-way crossover study was conducted in 12 healthy Korean male subjects. They were allocated to one of the two treatment sequences of RT and TR (R, reference drug, Trast® patch; T, test drug, Murupe® patch). Each patch was applied to the subject once during 48 hours. Serial blood samples were collected up to 72 hours after removing the patch and plasma concentrations were determined by high performance liquid chromatography. Safety was monitored and the skin irritation potential was assessed.
Results:
The plasma concentration - time profile during 48 hours showed an exponential increase in both of two patch products. Mean Cmax and AUClast values were not statistically different between two patch groups. Mean AUC[0-48h] was lower in Murupe® patch group than that in Trast® patch group; 806.4 and 1196.5 ng·h/mL However, the mean AUC[48-120h] value tended to be higher in Murupe® patch group, implying more delayed excretion than in Trast® patch group; 2724.7 ng·h/mL and 1989.2 ng·h/mL. The overall results of skin irritation potential test showed no clinically significant differences between two patch groups.
Go to : 
REFERENCES
1. Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998; 47:487–504.
2. Day RO, Brooks PM. Variations in response to non-steroidal anti-inflammatory drugs. Br J Clin Pharmacol. 1987; 23(6):655–658.

3. Gennaro AR. Remington’s Pharmaceutical Sciences. 18th ed. Mack Publishing Company;1990. p. 1116.
4. Skjodt NM, Davies NM. Clinical pharmacokinetics of lornoxicam. A short half-life oxicam. Clin Pharmacokinet. 1998; 34(6):421–428.
5. Bombardier C. An evidence-based evaluation of the gastrointestinal safety of coxibs. Am J Cardiol. 2002; 89(6A):3D–9D.

6. Bijlsma JW. Patient benefit risk in arthritis-a rheumatologist’s perspective. Rheumatology. 2010; 49:ii11–ii17.
7. Dahl SL, Ward JR. Pharmacology, clinical efficacy, and adverse effects of piroxicam, a new nonsteroidal anti-inflammatory agent. Pharmacotherapy. 1982; 2(2):80–90.

8. Fourtillan JB, Girault J. Piroxicam plasma concentrations following repeated topical application of a piroxicam 0.5 % gel. Drug Invest. 1992; 4(5):435–440.
9. EMEA, EMEA web sites on PRESS RELEASE. Key expected results. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/11/WC500012655.pdf. [Online] (last visited on 5 Feb 2013).
10. Derry S, Moore RA, Rabbie R. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2012; 9:CD007400.

11. Allegrini A, Nuzzo L, Pavone D, Tavella- Scaringi A, Giangreco D, Bucci M, Toniato E, Mezzetti A, Martinotti S, Comuzio S, Di Grigoli M, Bonani S. Efficacy and safety of piroxicam patch versus piroxicam cream in patients with lumbar osteoarthritis. A randomized, placebo-controlled study. Arzneimittelforschung. 2009; 59(8):403–409.
12. Campione E, Diluvio L, Paterno EJ, Chimenti S. Topical treatment of actinic keratoses with Piroxicam 1 % gel: a preliminary open-label study utilizing a new clinical score. Am J Clin Dermatol. 2010; 11(1):45–50.
13. Dixit M, Kini AG, Kulkarni PK. Preparation and characterization of microparticles of piroxicam by spray drying and spray chilling methods. Res Pharm Sci. 2010; 5(2):89–97.
14. van Haselen RA, Fisher PA. A randomized controlled trial comparing topical piroxicam gel with a homeopathic gel in osteoarthritis of the knee. Rheumatology (Oxford). 2000; 39(7):714–719.

15. Roy SD, Gutierrez M, Flynn GL, Cleary GW. Controlled transdermal delivery of fentanyl: characterizations of pressure-sensitive adhesives for matrix patch design. J Pharm Sci. 1996; 85(5):491–495.

16. KFDA. Korean Good Clinical Practice (KGCP). 2011.7.19. http://www.kfda.go.kr/index.kfda?mid=95&seq=3566&cmd=v. [Online] (last visited on 5 Feb 2013).
17. Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basic for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vitro bioavailability. Pharm Res. 1995; 12(3):413–420.
18. Dixit M, Kulkarni PK. Lyophilization monophase solution technique for improvement of the solubility and dissolution of piroxicam. Res Pharm Sci. 2012; 7(1):13–21.
19. Lee JJ, Kim SY. Non-steroidal anti-inflammatory drug patch having improved penetration rate thereof by concentration gradient in matrix. patent registered, 10-2012-0054135 (2012).
20. Cheong HA, Choi HK. Enhanced percutaneous absorption of piroxicam via salt formation with ethanolamines. Pharm Res. 2002; 19(9):1375–1380.
21. Komatsu T, Sakurada T. Comparison of the efficacy and skin permeability of topical NSAID preparations used in Europe. Eur J Pharm Sci. 2012; 47(5):890–895.

23. Plessis JD, Stefaniak A, Eloff F, John S, Agner T, Chou TC, Nixon R, Steiner M, Franken A, Kudla I, Holness L. International guidelines for the in vivo assessment of skin properties in non-clinical settings: Part 2. Transepidermal water loss and skin hydration. Skin Res Technol. 2013; Jan 19. doi: 10.1111/srt.12037.
Go to : 
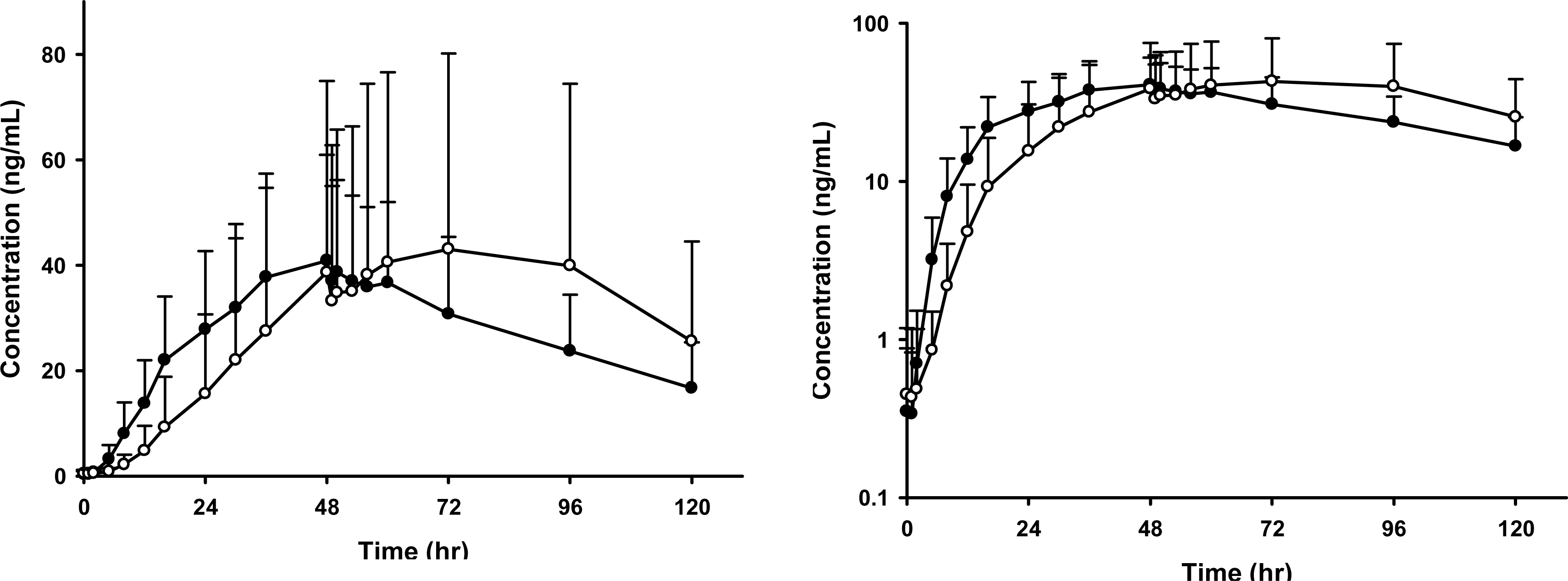 | Figure 1.Mean plasma piroxicam concentration-time profile by treatment group. Bars represent standard deviations (Left: linear scale, right: log-linear scale. ∘ : Murupe® patch. • : Trast® patch). |
Table 1.
Demographic data of subjects
Table 2.
Adverse event profile
Table 3.
Evaluation of skin irritation test taken from two sides of the attachment sites
Table 4.
Summary of pharmacokinetic parameters




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download