Abstract
Background:
Methods:
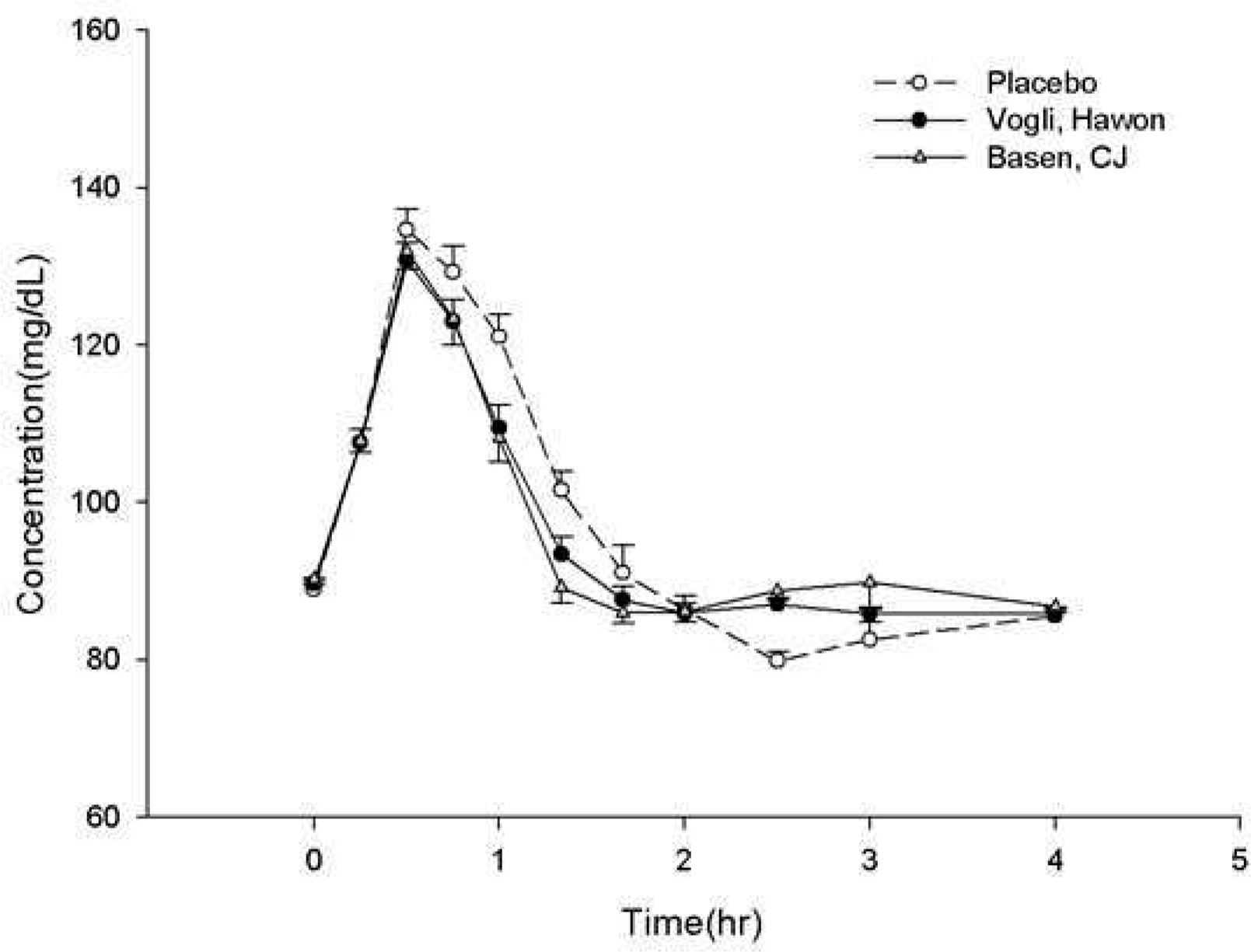
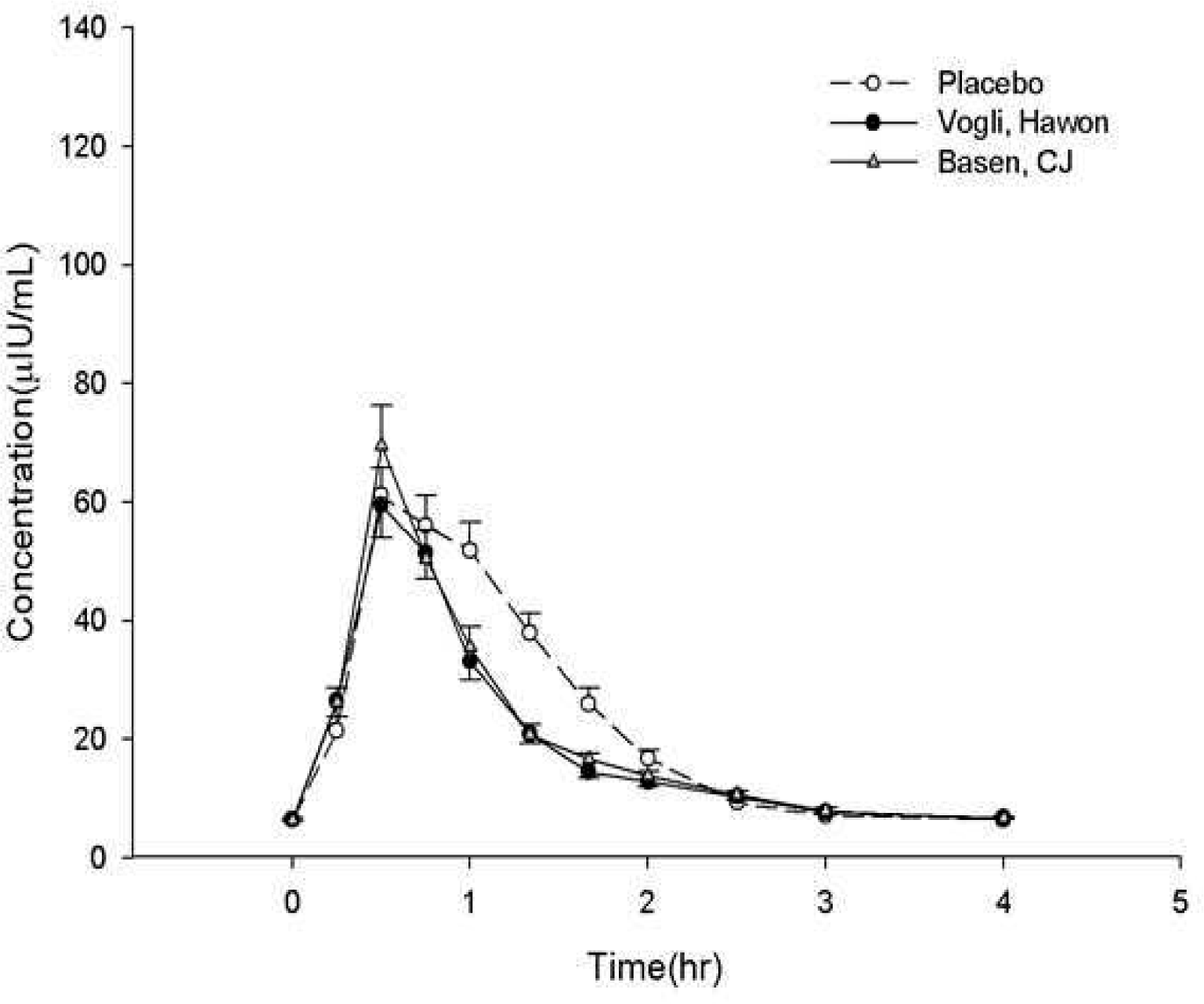
Results:
REFERENCES
Table 1.
Table 2.
Table 3.
* Maximum serum glucose concentration. †Area under the serum glucose versus time curve from dosing to 2 hours post-dose. ‡ Area under the serum glucose versus time curve from dosing to 4 hours post-dose. § Maximum serum insulin concentration. ¶ Area under the serum insulin versus time curve from dosing to 2 hours post-dose. ¶ Area under the serum insulin versus time curve from dosing to 4 hours post-dose.
Table 4.
* Maximum serum glucose concentration. † Area under the serum glucose versus time curve from dosing to 2 hours post-dose. ‡ Area under the serum glucose versus time curve from dosing to 4 hours post-dose. § Maximum serum insulin concentration. ¶ Area under the serum insulin versus time curve from dosing to 2 hours post-dose. ¶ Area under the serum insulin versus time curve from dosing to 4 hours post-dose.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download