Abstract
Background:
Bivalirudin is a direct thrombin inhibitor for patients with unstable angina undergoing percutaneous coronary intervention.
Methods:
A sensitive liquid chromatography-tandem mass spectrometry (LC/MS/MS) method was developed and validated for the determination of bivalirudin, in human plasma using nafarelin as internal standard (IS). Chromatographic separation was performed using a Shiseido MG3 mm column (2.0 × 50 mm) with a gradient mobile phase consisting of water and acetonitrile containing 0.1 % formic acid at a flow rate of 0.4 mL/min, and total run time was within 5 min. Detection and quantification was performed by the mass spectrometer using a multiple reaction-monitoring mode at m/z 1091.0 → 650.3 for bivalirudin, and m/z 662.1 → 249.3 for IS.
Go to : 
REFERENCES
2. Lincoff AM, Bittl JA, Harrington RA, Feit F, Kleiman NS, Jackman JD, Sarembock IJ, Cohen DJ, Spriggs D, Ebrahimi R, Keren G, Carr J, Cohen EA, Betriu A, Desmet W, Kereiakes DJ, Rutsch W, Wilcox RG, de Feyter PJ, Vahanian A, Topol EJ, Investigators R. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA. 2003; 289(7):853–863.
3. EMEA. Scientific discussion for the approval of Bivalirudin [cited 2013 31 Oct]. Available from. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000562/WC500025072.pdf.
4. FDA. NDA 020873 Angiomax (Bivalirudin): Clinical Pharmacology Biopharmaceutics Review. Rockville, MD: Department of Health and Human Services, US Food and Drug Administration;2003.
5. Robson R, White H, Aylward P, Frampton C. Bivalirudin pharmacokinetics and pharmacodynamics: effect of renal function, dose, and gender. Clin Pharmacol Ther. 2002; 71(6):433–439.

6. Lidon RM, Theroux P, Juneau M, Adelman B, Maraganore J. Initial experience with a direct antithrombin, Hirulog, in unstable angina. Anticoagulant, antithrombotic, and clinical effects. Circulation. 1993; 88(4 Pt 1):1495–1501.

7. Topol EJ, Bonan R, Jewitt D, Sigwart U, Kakkar VV, Rothman M, de Bono D, Ferguson J, Willerson JT, Strony J, et al. Use of a direct antithrombin, hirulog, in place of heparin during coronary angioplasty. Circulation. 1993; 87(5):1622–1629.

8. Reed MD, Bell D. Clinical pharmacology of bivalirudin. Pharmacotherapy. 2002; 22(6 Pt 2): 105S–111S.

9. Anand SX, Viles-Gonzalez JF, Mahboobi SK, Heerdt PM. Bivalirudin utilization in cardiac surgery: shifting anticoagulation from indirect to direct thrombin inhibition. Can J Anaesth. 2011; 58(3):296–311.

10. Shammas NW. Bivalirudin: pharmacology and clinical applications. Cardiovasc Drug Rev. 2005; 23(4):345–360.

11. Pan G, Wang X, Huang Y, Gao X, Wang Y. Development and validation of a LC-MS/MS method for determination of bivalirudin in human plasma: Application to a clinical pharmacokinetic study. J Pharm Biomed Anal. 2010; 52(1):105–109.

12. Farthing D, Larus T, Fakhry I, Gehr T, Prats J, Sica D. Liquid chromatography method for determination of bivalirudin in human plasma and urine using automated ortho-phthalaldehyde derivatization and fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2004; 802(2):355–359.

13. Maraganore JM, Bourdon P, Jablonski J, Ramachandran KL, Fenton JW 2nd. Design and characterization of hirulogs: a novel class of bivalent peptide inhibitors of thrombin. Biochemistry. 1990; 29(30):7095–7101.

14. Zhang X, Nieforth K, Lang JM, Rouzier-Panis R, Reynes J, Dorr A, Kolis S, Stiles MR, Kinchelow T, Patel IH. Pharmacokinetics of plasma enfuvirtide after subcutaneous administration to patients with human immunodeficiency virus: Inverse Gaussian density absorption and 2-compartment disposition. Clin Pharmacol Ther. 2002; 72(1):10–19.
15. Douglas SA. Human urotensin-II as a novel cardiovascular target: ‘heart’ of the matter or simply a fishy ‘tail’? Curr Opin Pharmacol. 2003; 3(2):159–167.

16. John H, Walden M, Schafer S, Genz S, Forssmann WG. Analytical procedures for quantification of peptides in pharmaceutical research by liquid chromatography-mass spectrometry. Anal Bioanal Chem. 2004; 378(4):883–897.

17. FDA. Bioanalytical Method Validation (Draft Guidance). Rockville, MD: Department of Health and Human Services, US Food and Drug Administration;2013.
Go to : 
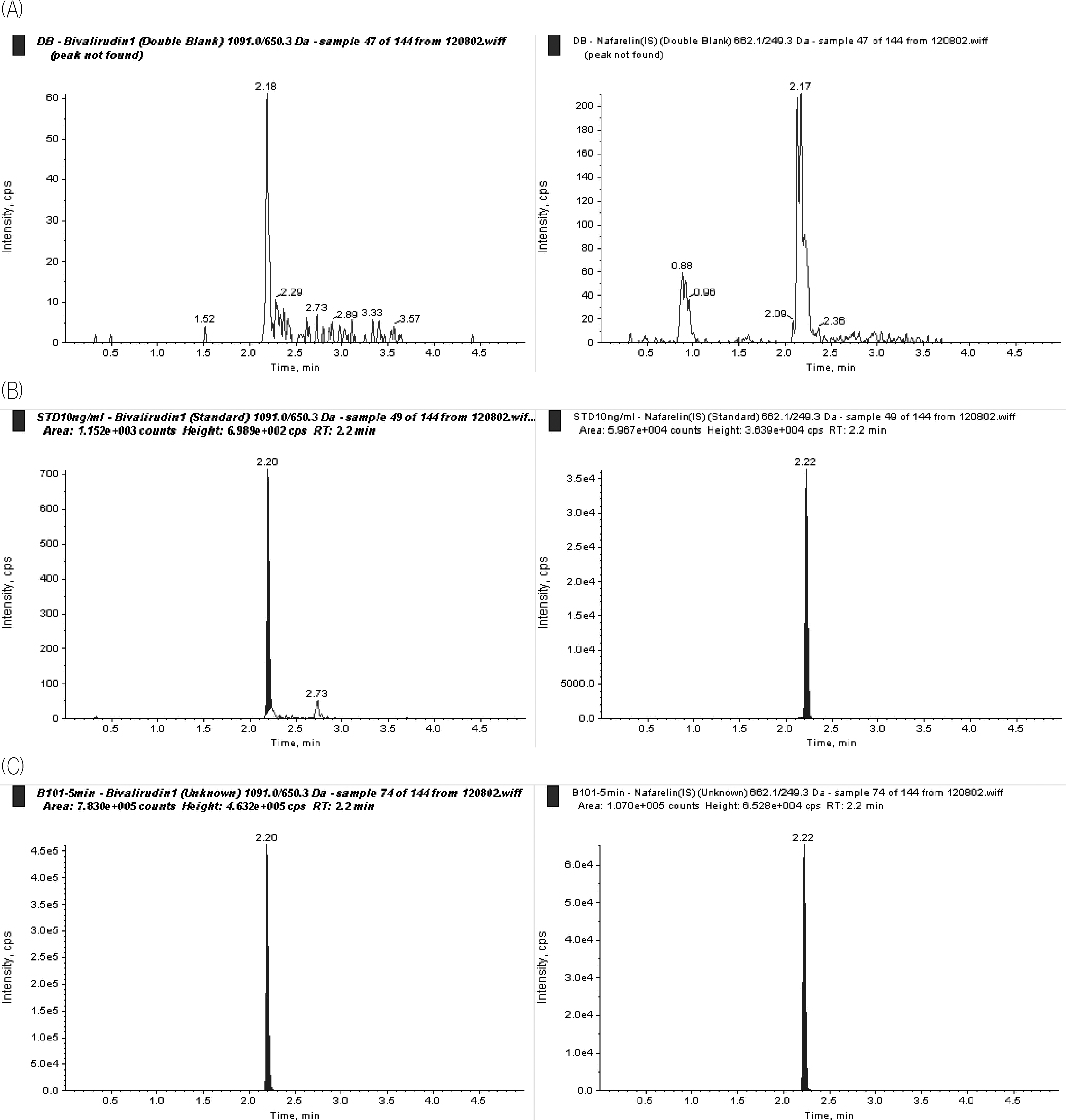 | Figure 1.Chromatograms of (A) a human blank plasma sample; (B) a human plasma sample spiked with 10 ng/mL of bivalirudin and IS (nafarelin); (C) a subject’s plasma sample at 5 min after intravenous bolus administration of bivalirudin 0.375 mg/kg. |
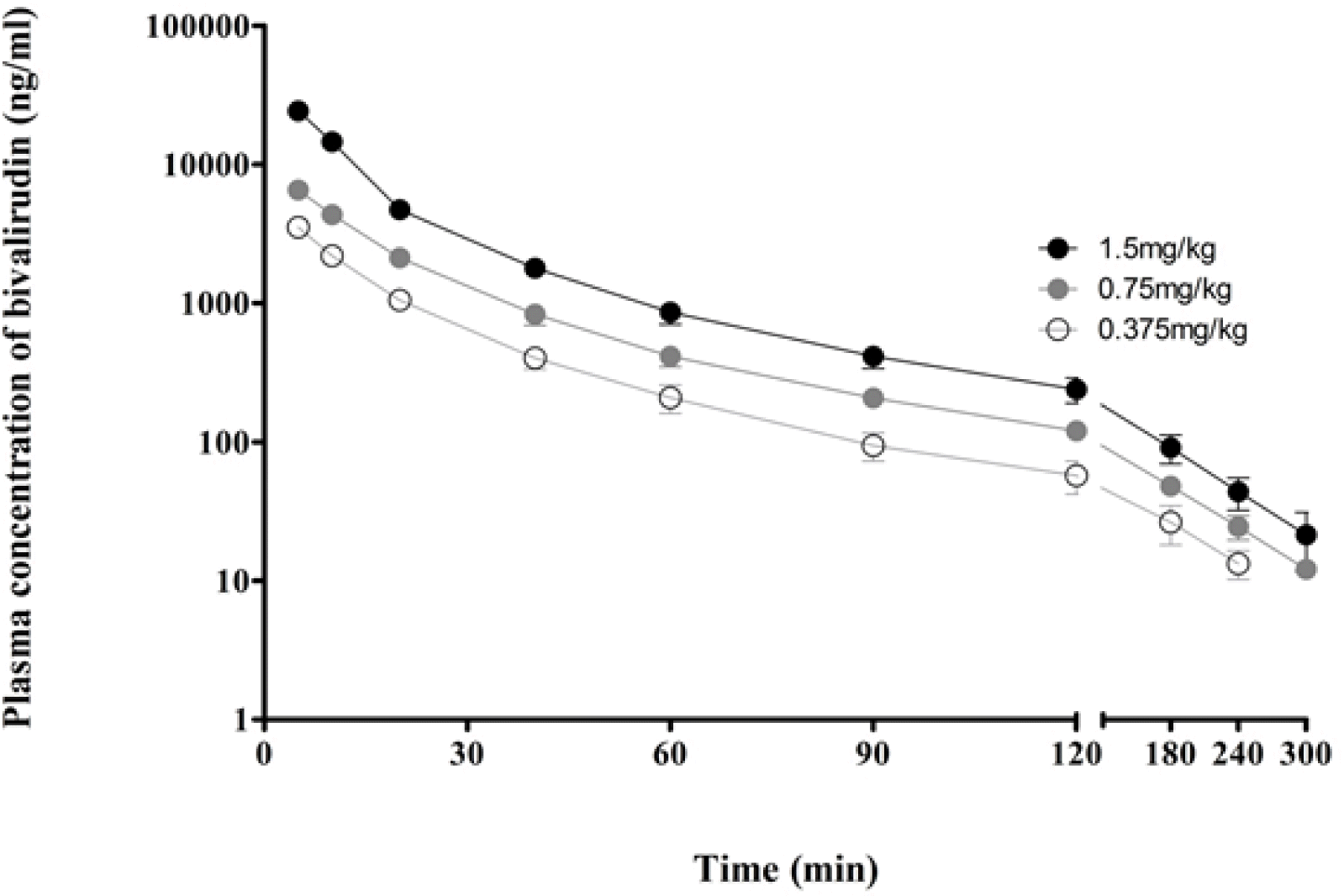 | Figure 2.Mean plasma concentration time curves of bivalirudin after single intravenous bolus injection. Data are expressed as mean ± SD (each group, n=6). |
Table 1.
Intra- and inter-day precision and accuracy data for this assay of bivalirudin in human plasma (n=5)
Table 2.
Stability of bivalirudin in human plasma




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download