Abstract
Background:
JES9501 is dehydroevodiamine, the extract of Evodia rutaecarpa, expected to be a new therapeutic for Alzheimer disease. This study aims to investigate the pharmacokinetics (PK), pharmacodynamics (PD) and safety of JES9501 after single or multiple dosing.
Methods:
A double-blind, randomized, placebo-controlled, dose ascending, parallel study was conducted in healthy subjects. A single dose of JES9501 50 • 100 • 200 • 400 or 800 mg and multiple doses of JES9501 100 - 200 or 400 mg once-daily for 7days was administered. Serial blood and urine samples for PK evaluation were collected. Acetylcholinesterase (AChE) activity was measured for PD evaluation in multiple dose group.
Results:
In the single dose study, means of dose-normalized peak concentration (Cmax) of 100 • 200 • 400 and 800 mg dose group are comparable except 50 mg dose group. Means of dose-normalized area under the plasma concentration-time curve (AUC) from dosing to the last quantifiable concentration of corresponding dose group were similar. At steady state in the multiple dose study, means of dose-normalized Cmax and AUC for dosing interval of 100 • 200 and 400 mg dose group decreased as the dose increased, however those were not relevant. There was no significant difference of AChE activity between three dosage groups and placebo group. Adverse events related to study drug were all mild and there were no remarkable findings.
REFERENCES
1. Seoul National University Hospital. Nationwide Study on the Prevalence of Dementia in Korean Elders. 2008; (Korean).
2. Han SH. Novel Pharmacotherapies for Alzheimer’s Disease. J Korean Med Assoc. 2009; 52(11):1059–1068. (Korean).

3. Williams P, Sorribas A, Howes M J. Natural products as a source of Alzheimer’s drug leads. Nat Prod Rep. 2011; 28(1):48–77.

4. Lee JY, Cha MR, Chois CW, Kim YS, Lee BH, Ryu SY. Cholinesterase Inhibitors Isolated from the Fruits Extract of Evodia officinalis. Kor J Pharmacogn. 2012; 43(2):122–126. (Korean).
5. Lee SW, Hwang BY, Kim SE, Kim HM, Kim YH, Lee KS, Lee JJ, Ro JS. Isolation of modulators for multdrug resistance from the fruits of Evodia officinalis. Kor J Pharmacogn. 1995; 26(4):344–348. (Korean).
6. Yun HJ, Heo SK, Lee YT, Park WH, Park SD. Anti-inflammatory effect of Evodia officinalis Dode in mouse macrophage and human vascular endotherial cells. Kor J Herbology. 2008; 23(1):29–38. (Korean).
7. ORHAN İ, ŞENER B. Acetylcholinesterase Inhibitors from Natural Resources. FABAD J Pharm Sci. 2003; 28:51–58.
8. Jeil Pharmaceutical Co., Ltd.Investigator’s Brochure. 2003; (Korean).
9. FDA. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Guidance for Industry. 2005.
10. Mosca A, Ghezzi A, Luzzana M, Paleari R, Imbimbo BP. Pharmacodynamic monitoring of eptastigmine in capillary blood. Eur J Clin Pharmacol. 1996; 50(5):425–427.

11. Worek F, Mast U, Kiderlen D, Diepold C, Eyer P. Improved determination of acetylcholinesterase activity in human whole blood. Clin Chim Acta. 1999; 288(1-2):73–90.

12. Lotti M. Cholinesterase inhibition: complexities in interpretation. Clin Chem. 1995; 41(12):1814–1818.

13. Augustinsson K-B. The normal variation of human blood cholinesterase activity. Acta Physiol Scand. 1955; 35(1):40–52.
14. Sidell FR, Kaminskis A. Influence of age, sex, and oral contraceptives on human blood cholinesterase activity. Clin Chem. 1975; 21(10):1393–1395.

15. Biagioni MC, Galvin JE. Using biomarkers to improve detection of Alzheimer’s disease. Neurodegener Dis Manag. 2011; 1(2):127–139.

16. Coley N, Andrieu S, Delrieu J, Voisin T, Vellas B. Biomarkers in Alzheimer’s disease: not yet surrogate endpoints. Ann N Y Acad Sci. 2009; 1180:119–124.
17. Galasko D, Golde TE. Biomarkers for Alzheimer’s disease in plasma, serum and blood-conceptual and practical problems. Alzheimers Res Ther. 2013; 5(2):10.
Figure 1.
Mean JES9501 plasma concentration-time profiles after single oral administration (Left: linear scale, Right: log-linear scale, bar: standard deviation).
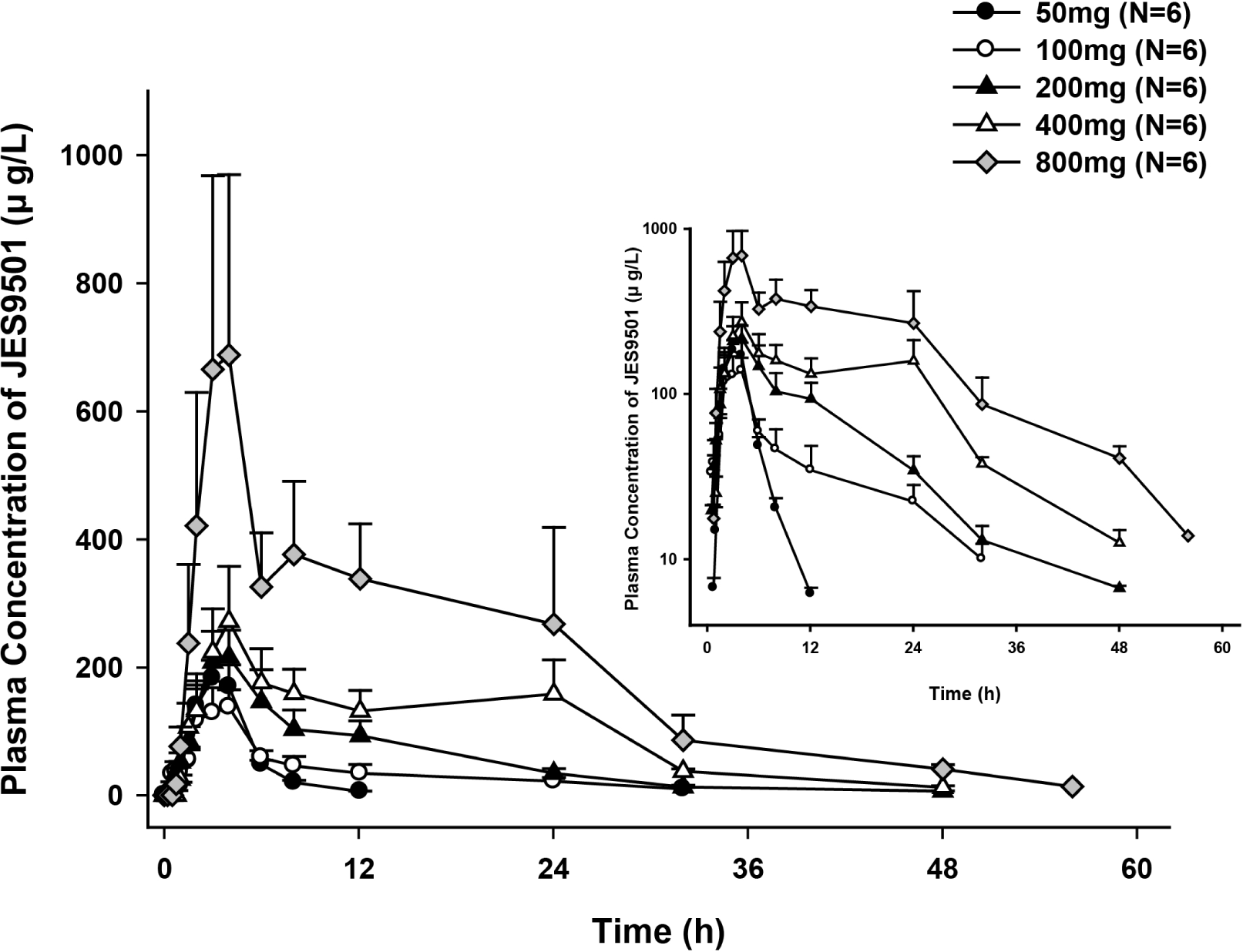
Figure 2.
Mean JES9501 plasma concentration-time profiles after multiple oral administration of JES9501 once daily for 7days (Left: linear scale, Right: log-linear scale, bar: standard deviation).
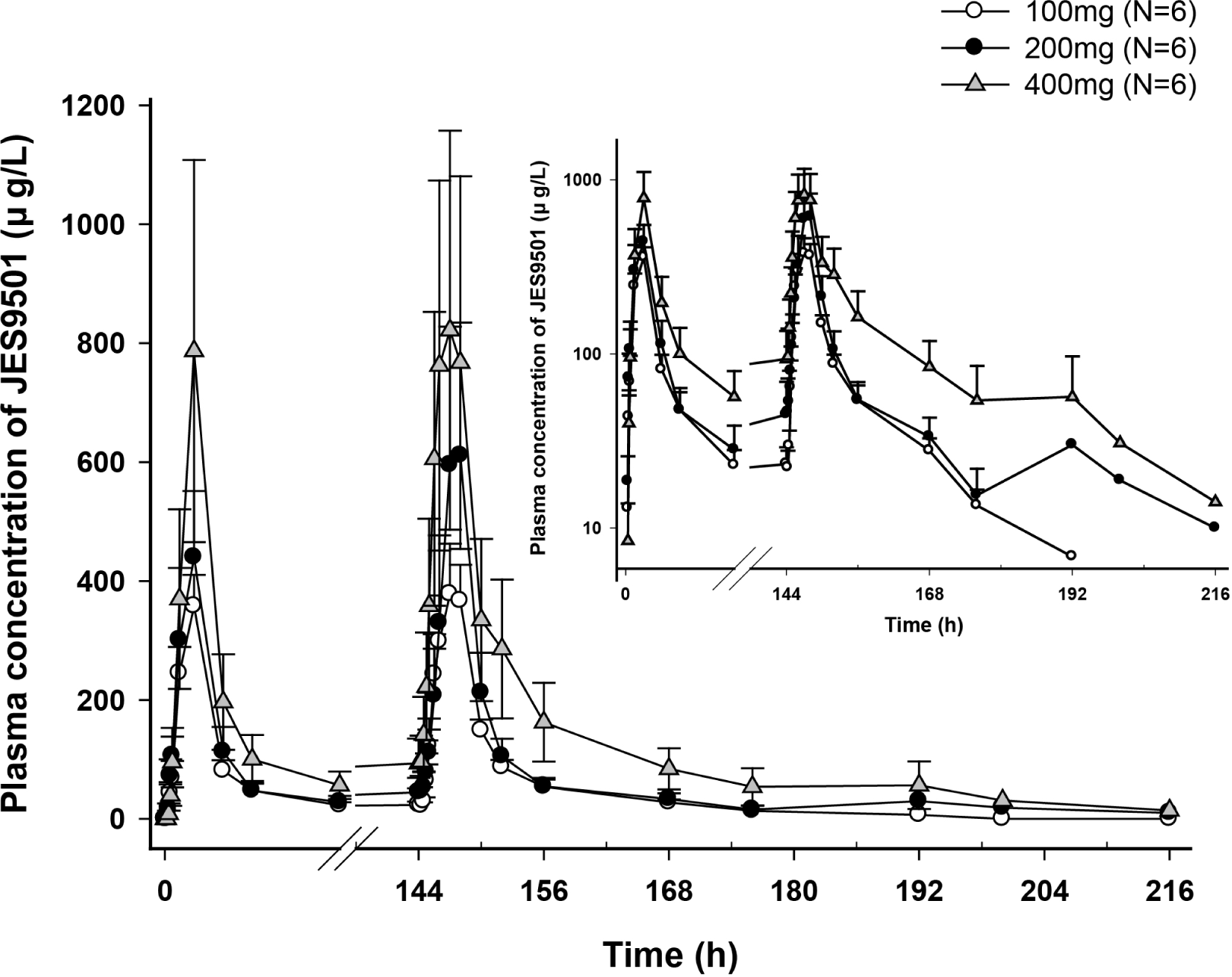
Figure 3.
Acetylcholinesterase activity-time profile at predose, Day 4, Day 7 and Day 9 after multiple oral administrations of JES9501 once daily for 7days (bar: standard deviation); Acetylcholinesterase was measured in Placebo group (N=5), and 200 mg group (N=2) on day 4.
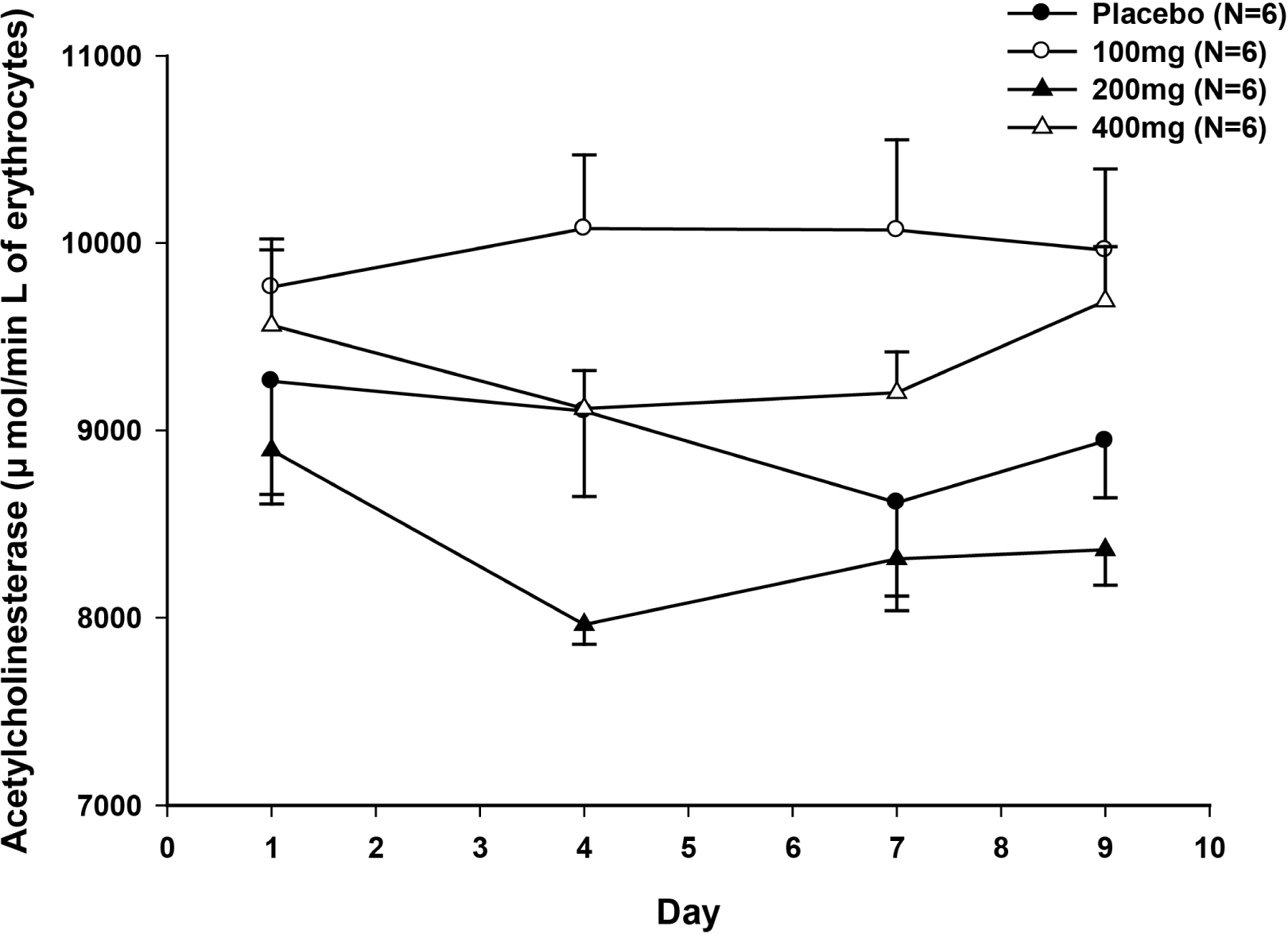
Table 1.
Pharmacokinetic parameters of JES9501 after single oral administration of JES9501*
Table 2.
Pharmacokinetic parameters of JES9501 on Day 7 after multiple oral administration of JES9501 once daily for 7 days*




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download