1. Logie J, Clifford GM, Farmer RDT. Incidence, prevalence and management of lower urinary tract symptoms in men in the UK. BJU Int. 2005; 95(4):557–562.

2. Glasser DB, Carson C 3rd, Kang JH, Laumann EO. Prevalence of storage and voiding symptoms among men aged 40 years and older in a US population-based study: results from the Male Attitudes Regarding Sexual Health study. Int J Clin Pract. 2007; 61(8):1294–1300.
3. Andersson KE. Alpha-adrenoceptors and benign prostatic hyperplasia: basic principles for treatment with alpha-adrenoceptor antagonists. World J Urol. 2002; 19(6):390–396.
4. Abrams P, Andersson KE. Muscarinic receptor antagonists for overactive bladder. BJU Int. 2007; 100(5):987–1006.

5. Naslund MJ, Miner M. A review of the clinical efficacy and safety of 5α-reductase inhibitors for the enlarged prostate. Clin Ther. 2007; 29(1):17–25.

6. van Hoogdaelm EJ, Soeishi Y, Matsushima H, Hiquchi S. Disposition of the selective α 1A-adrenoreceptor antagonist tamsulosin in humans: comparison with data from interspecies scaling. J Pharm Sci. 1997; 86(10):1156–1161.
7. Michel MC, Grübbel B, Taguchi K, VerfÜrth F, Otto T, KrÖpfl D. Drugs for treatment of benign prostatic hyperplasia: affinity comparison at cloned alpha 1-adrenoceptor subtypes and in human prostate. J Auton Pharmacol. 1996; 16(1):21–28.
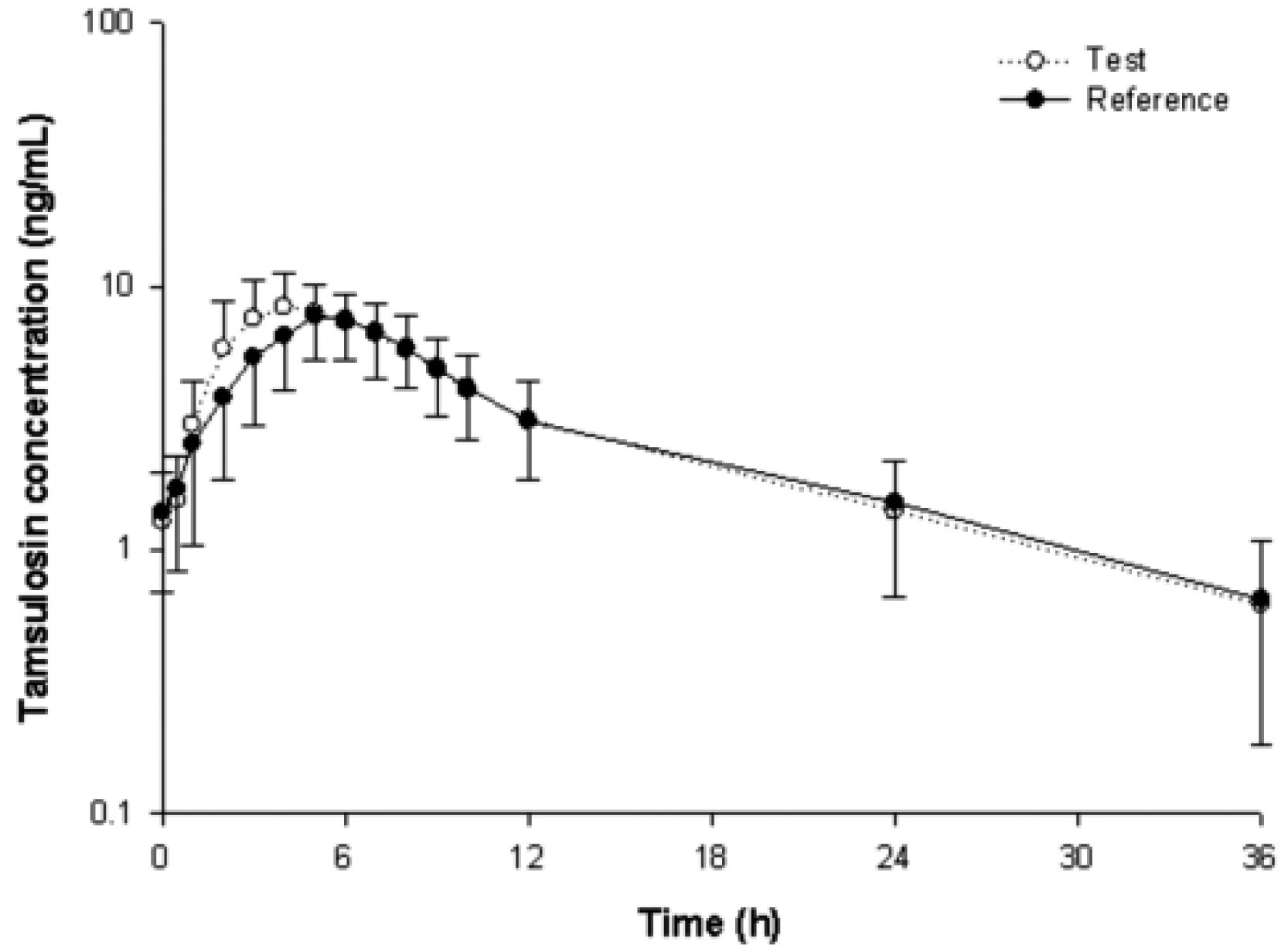
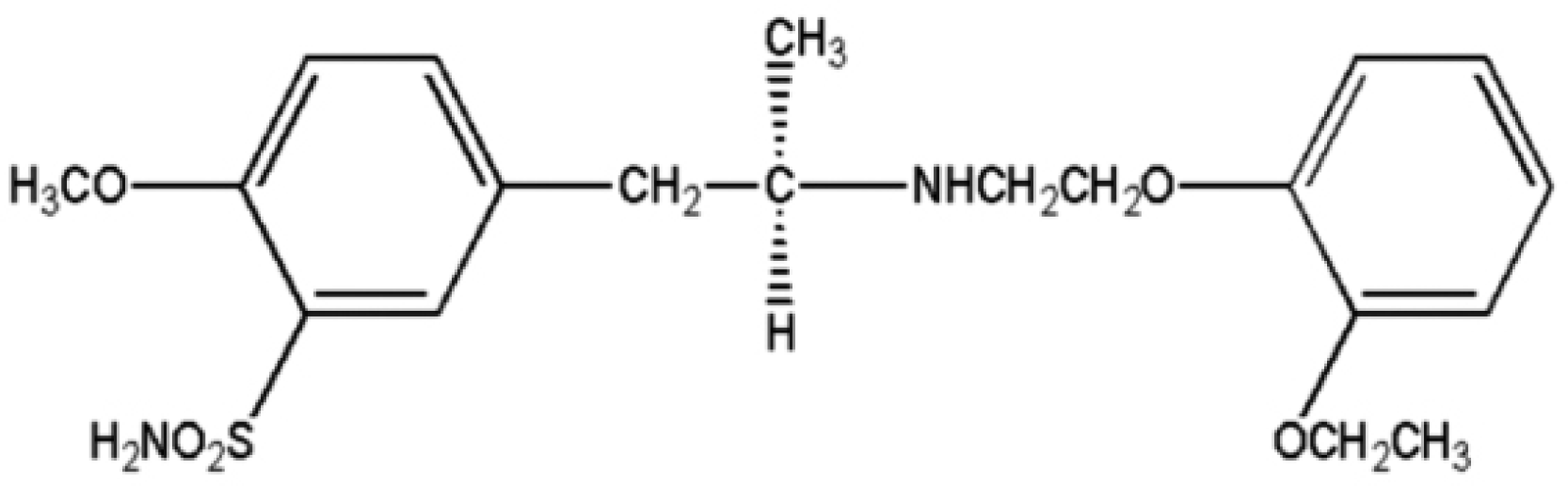
8. Prasaja B, Harahap Y, Lusthom W, Seitiawan EC, Ginting MB, Hardiyanti , Lipin . A bioequivalence study of two tamsulosin sustained-release tablets in Indonesian healthy volunteers. Eur J Drug Metab Pharmacokinet. 2011; 36(2):109–113.

9. Lyseng-Williamson KA, Jarvis B, Wagstaff AJ. Tamsulosin: an update of its role in the management of lower urinary tract symptoms. Drugs. 2002; 62(1):135–167.
10. Franco-Salinas G, de la Rosette JJMCH, Michel MC. Pharmacokinetics and pharmacodynamics of tamsulosin in its modified-release and oral controlled absorption system formulations. Clin Pharmacokinet. 2010; 49(3):177–188.

11. Koiso K, Akaza H, Kikuchi K, Aoyagi K, Ohba S, Miyazaki M, Ito M, Sueyoshi T, Matsushima H, Kamimura H, Watanabe T, Higuchi S. Pharmacokinetics of tamsulosin hydrochloride in patients with renal impairment: effects of α1-acid glycoprotein. J Clin Pharmacol. 1996; 36(11):1029–1038.
12. Matsushima H, Kamimura H, Soeishi Y, Watanabe T, Higuchi S, Tsunoo M. Pharmacokinetics and plasma protein binding of tamsulosin hydrochloride in rats, dogs, and humans. Drug Metab Dispos. 1998; 26(3):240–245.
13. Kamimura H, Oishi S, Matsushima H, Watanabe T, Higuchi S, Hall M, Wood SG, Chasseaud LF. Identification of cytochrome P450 isozymes involved in metabolism of the α1-adrenoceptor blocker tamsulosin in human liver microsomes. Xenobiotica. 1998; 8(10):909–922.
14. Taguchi K, Saitoh M, Sato S, Asano M, Michel MC. Effects of tamsulosin metabolites at alpha-1 adrenoceptor subtypes. J Pharmacol Exp Ther. 1997; 280(1):1–5.
15. Wolzt M, Fabrizii V, Dorner GT, Zanaschka G, Leufkens P, Krauwinkel WJ, Eichler HG. Pharmacokinetics of tamsulosin in subjects with normal and varying degrees of impaired renal function: an open-label single-dose and multiple-dose study. Eur J Clin Pharmacol. 1998; 54(4):367–373.

16. Miyazawa Y, Blum RA, Schentag JJ, Kamimura H, Matsushima H, Swarz H, Ito Y. Pharmacokinetics and safety of tamsulosin in subjects with normal and impaired renal or hepatic function. Curr Ther Res. 2001; 62(9):603–621.

17. Taguchi K, Schäfers RF, Michel MC. Radio-receptor assay analysis of tamsulosin and terazosin pharmacokinetics. Br J Clin Pharmacol. 1998; 45(1):49–55.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download