Abstract
The Korean Diabetes Association (KDA) recently updated the Clinical Practice Guidelines on antihyperglycemic agent therapy for adult patients with type 2 diabetes mellitus (T2DM). In combination therapy of oral hypoglycemic agents (OHAs), general recommendations were not changed compared to those of 2015 KDA guidelines. The committee of Clinical Practice Guideline of KDA has extensively reviewed and discussed the results of meta-analyses and systematic reviews of the effectiveness and safety of OHAs and many clinical trials on Korean patients with T2DM for the update of guidelines. All OHAs were effective when added to metformin or metformin and sulfonylurea, although the effects of each agent on body weight and hypoglycemia were different. Therefore, selection of a second agent as a metformin add-on therapy or a third agent as a metformin and sulfonylurea add-on therapy should be based on the patient's clinical characteristics and the efficacy, side effects, cardiovascular benefit, risk of hypoglycemia, effect on body weight, patient preference, and combined comorbidity.
References
1. DeFronzo RA, Lewin A, Patel S, Liu D, Kaste R, Woerle HJ, Broedl UC. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015; 38:384–93.

2. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, DeFronzo RA, Einhorn D, Fonseca VA, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez GE. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm-2017 executive summary. Endocr Pract. 2017; 23:207–38.
3. Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, Maggo J, Gray V, De Berardis G, Ruospo M, Natale P, Saglimbene V, Badve SV, Cho Y, Nadeau-Fredette AC, Burke M, Faruque L, Lloyd A, Ahmad N, Liu Y, Tiv S, Wiebe N, Strippoli GF. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a metaanalysis. JAMA. 2016; 316:313–24.
4. Zhou JB, Bai L, Wang Y, Yang JK. The benefits and risks of DPP4-inhibitors vs. sulfonylureas for patients with type 2 diabetes: accumulated evidence from randomised controlled trial. Int J Clin Pract. 2016; 70:132–41.
5. Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, Chu Y, Iyoha E, Segal JB, Bolen S. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and metaanalysis. Ann Intern Med. 2016; 164:740–51.
6. Amate JM, Lopez-Cuadrado T, Almendro N, Bouza C, Saz-Parkinson Z, Rivas-Ruiz R, Gonzalez-Canudas J. Effectiveness and safety of glimepiride and iDPP4, associated with metformin in second line pharmacotherapy of type 2 diabetes mellitus: systematic review and metaanalysis. Int J Clin Pract. 2015; 69:292–304.

7. Mishriky BM, Cummings DM, Tanenberg RJ. The efficacy and safety of DPP4 inhibitors compared to sulfonylureas as add-on therapy to metformin in patients with Type 2 diabetes: a systematic review and metaanalysis. Diabetes Res Clin Pract. 2015; 109:378–88.

8. Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and metaanalysis. Diabetologia. 2013; 56:696–708.

9. Storgaard H, Gluud LL, Bennett C, Grøndahl MF, Christensen MB, Knop FK, Vilsbøll T. Benefits and harms of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes: a systematic review and metaanalysis. PLoS One. 2016; 11:e0166125.

10. Buschur E, Sarma AV, Pietropaolo M, Dunn RL, Braffett BH, Cleary PA, Cowie C, Larkin ME, Wessells H, Nathan DM, Kim C. DCCT/EDIC Research Group. Self-reported autoimmune disease by sex in the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabetes Care. 2014; 37:e28–9.

11. Zinman B, Genuth S, Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study: 30th anniversary presentations. Diabetes Care. 2014; 37:8.

12. Ha KH, Kim DJ. Current status of managing diabetes mellitus in Korea. Korean J Intern Med. 2016; 31:845–50.

13. Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013; 382:941–50.

14. Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC. EMPA-REG H2H-SU trial investigators. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014; 2:691–700.

15. Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, Capuano G, Canovatchel W. Canagliflozin DIA 2001 Study Group. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012; 35:1232–8.

16. Henry RR, Rosenstock J, Edelman S, Mudaliar S, Chalamandaris AG, Kasichayanula S, Bogle A, Iqbal N, List J, Griffen SC. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care. 2015; 38:412–9.

17. Kim JH, Kim SS, Baek HS, Lee IK, Chung DJ, Sohn HS, Bae HY, Kim MK, Park JH, Choi YS, Kim YI, Hahm JR, Lee CW, Jo SR, Park MK, Lee KJ, Kim IJ. Comparison of vildagliptin and pioglitazone in Korean patients with type 2 diabetes inadequately controlled with metformin. Diabetes Metab J. 2016; 40:230–9.

18. Jin SM, Park CY, Cho YM, Ku BJ, Ahn CW, Cha BS, Min KW, Sung YA, Baik SH, Lee KW, Yoon KH, Lee MK, Park SW. Lobeglitazone and pioglitazone as add-ons to metformin for patients with type 2 diabetes: a 24-week, multicentre, randomized, double-blind, parallel-group, active-controlled, phase III clinical trial with a 28-week extension. Diabetes Obes Metab. 2015; 17:599–602.
19. Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokán M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005; 366:1279–89.

20. Seong JM, Choi NK, Shin JY, Chang Y, Kim YJ, Lee J, Kim JY, Park BJ. Differential cardiovascular outcomes after dipeptidyl peptidase-4 inhibitor, sulfonylurea, and pioglitazone therapy, all in combination with metformin, for type 2 diabetes: a population-based cohort study. PLoS One. 2015; 10:e0124287.

21. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G. ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006; 355:2427–43.

22. Mamza J, Mehta R, Donnelly R, Idris I. Important differences in the durability of glycaemic response among second-line treatment options when added to metformin in type 2 diabetes: a retrospective cohort study. Ann Med. 2016; 48:224–34.

23. Kahn SE, Lachin JM, Zinman B, Haffner SM, Aftring RP, Paul G, Kravitz BG, Herman WH, Viberti G, Holman RR. ADOPT Study Group. Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes. 2011; 60:1552–60.

24. Downes MJ, Bettington EK, Gunton JE, Turkstra E. Triple therapy in type 2 diabetes; a systematic review and network metaanalysis. PeerJ. 2015; 3:e1461.

25. Lee CM, Woodward M, Colagiuri S. Triple therapy combinations for the treatment of type 2 diabetes-a network metaanalysis. Diabetes Res Clin Pract. 2016; 116:149–58.
26. Lozano-Ortega G, Goring S, Bennett HA, Bergenheim K, Sternhufvud C, Mukherjee J. Network metaanalysis of treatments for type 2 diabetes mellitus following failure with metformin plus sulfonylurea. Curr Med Res Opin. 2016; 32:807–16.

27. Mearns ES, Saulsberry WJ, White CM, Kohn CG, Lemieux S, Sihabout A, Salamucha I, Coleman CI. Efficacy and safety of antihyperglycaemic drug regimens added to metformin and sulphonylurea therapy in Type 2 diabetes: a network metaanalysis. Diabet Med. 2015; 32:1530–40.

28. Ahn CH, Han KA, Yu JM, Nam JY, Ahn KJ, Oh TK, Lee HW, Lee DH, Kim J, Chung CH, Park TS, Kim BJ, Park SW, Park HK, Lee KJ, Kim SW, Park JH, Ko KP, Kim CH, Lee H, Jang HC, Park KS. Efficacy and safety of gemigliptin, a dipeptidyl peptidase-4 inhibitor, in patients with type 2 diabetes mellitus inadequately controlled with combination treatment of metformin and sulphonylurea: a 24-week, multicentre, randomized, double-blind, placebo-controlled study (TROICA study). Diabetes Obes Metab. 2017; 19:635–43.
29. Hong AR, Lee J, Ku EJ, Hwangbo Y, Kim KM, Moon JH, Choi SH, Jang HC, Lim S. Comparison of vildagliptin as an add-on therapy and sulfonylurea dose-increasing therapy in patients with inadequately controlled type 2 diabetes using metformin and sulfonylurea (VISUAL study): a randomized trial. Diabetes Res Clin Pract. 2015; 109:141–8.

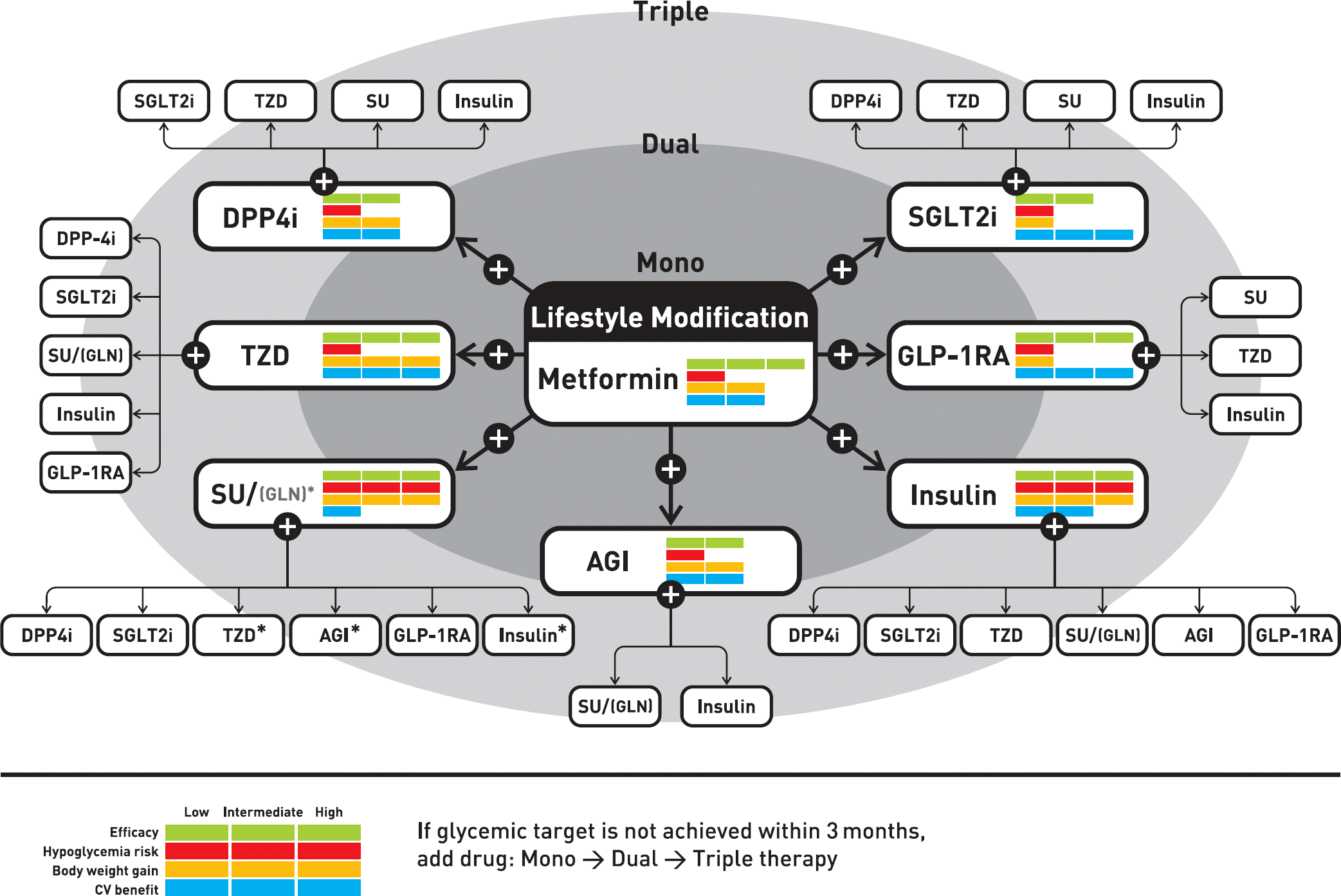
Fig. 1.
Antihyperglycemic therapy algorithm for adult patients with type 2 diabetes mellitus. SGLT2i, sodium glucose cotransporter 2 inhibitors; TZD, thiazolidinedione; SU, sulfonylurea; DPP4i, dipeptidyl peptidase 4 inhibitors; GLP-1RA, glucagon-like peptide 1 receptor agonists; AGI, alpha glucosidase inhibitors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download