Abstract
The primary causes of uncontrolled diabetes are poor life-style, infection, ischemic heart disease and inappropriate usage of oral anti-diabetic agents and insulin. Supplementary causes are stroke, acute pancreatitis and endocrine diseases. Multiple endocrine neoplasia type 1 (MEN 1) is an autosomal dominant syndrome characterized by primary hyperparathyroidism, pituitary neoplasia, and foregut lineage neuroendocrine tumors, and is associated with increased glucose levels. We present a case of a 69-year-old woman who had polyuria, polydipsia, weight loss and hyperglycemia over 6 months. She had hypertrophy of the face, hand, and foot, and active bleeding and large folds were observed in the stomach and duodenum upon esophagogastroduodenoscopy. She also had high levels of IGF-1 and gastrin and got the failure of growth hormone suppression after an oral glucose load (75 g). These findings suggested a diagnosis of acromegaly and gastrinoma, which was clinically diagnosed along with MEN 1. The patient improved glycemic control and symptoms after being treated with somatostatin analogues and insulin therapy over a 5-month follow-up period. Here, we report a case of MEN 1 in type 2 diabetes mellitus with a poorly controlled blood glucose level. Clinicians should consider endocrine disease in patients with poor glycemic control in diabetes.
Figures and Tables
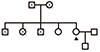 | Fig. 1A family pedigree of the patient (arrowhead). She refuses genetic test for MEN1 gene mutation. |
 | Fig. 2Sella magnetic resonance imaging (MRI). T2 weighted coronal view of sella MRI shows poorly enhancing mass in right sella, 1.6 × 1.1 × 1 cm. |
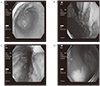 | Fig. 3Endoscopic findings show diffuse mucosal hypertrophy and enlarged gastric folds in the body of the stomach (A, B). There was a peptic ulcer with active bleeding in the 1st portion of the duodenum (C, D). |
 | Fig. 4Insulin-like growth factor-1 (IGF-1) and gastrin level during the treatment with long acting octretide (Sandostatin LAR®; Sandoz Pharma Ltd., Basel, Switzerland) 20 mg for 0, 4th week, 30 mg for 8th, 12th week, 20 mg 16th, 20th week. |
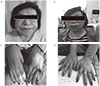 | Fig. 5Before treatment, an enlarged nose, protruding lips and thicker fingers were observed (A, C). After treatment, the patient's appearance is better (B, D). |
 | Fig. 6At 8 week, Patient wanted to switch insulin treatment to oral anti-diabetes drugs. During insulin treatment with long acting octretide, total insulin dose per day and HbA1c were decreased.IU, international unit.
|
References
2. Marx SJ, Wells SA. Multiple endocrine neoplasia. In : Melmed S, Polonsky K, Larsen PR, Kronenberg H, editors. Williams textbook of endocrinology. 12th ed. Philadelphia: Elsevier;2011. p. 1728–1767.
3. Morelli A, Falchetti A, Martineti V, Becherini L, Mark M, Friedman E, Brandi ML. MEN1 gene mutation analysis in Italian patients with multiple endocrine neoplasia type 1. Eur J Endocrinol. 2000; 142:131–137.

4. Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1(MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008; 29:22–32.

5. Stabile BE, Passaro E Jr. Recurrent pepti culcer. Gastroenterology. 1976; 70:124–135.
6. Hirschowitz BI. Zollinger-Ellison syndrome: pathogenesis, diagnosis, and management. Am J Gastroenterol. 1997; 92:4 Suppl. 44S–48S. discussion 49S-50S.
7. Orloff SL, Debas HT. Advances in the management of patients with Zollinger-Ellison syndrome. Surg Clin North Am. 1995; 75:511–524.

8. Bengtsson BA, Edén S, Ernest I, Odén A, Sjögren B. Epidemiology and long-term survival in acromegaly. A study of 166 cases diagnosed between 1955 and 1984. Acta Med Scand. 1988; 223:327–335.

9. Ezzat S, Melmed S. Clinical review 18: are patients with acromegaly at increased risk for neoplasia. J Clin Endocrinol Metab. 1991; 72:245–249.
10. Barzilay J, Heatley GJ, Cushing GW. Benign and malignant tumors in patients with acromegaly. Arch Intern Med. 1991; 151:1629–1632.

11. Ganda OP, Bachman ES. Diabetes secondary to endocrinopathies. In : Porte D, Sherwin RS, Baron A, Ellenberg M, Rifkin H, editors. Ellenberg and Rifkin's diabetes mellitus. 6th ed.New York: McGraw-Hill;2003. p. 425.
12. Melmed S. Medical progress: acromegaly. N Engl J Med. 2006; 355:2558–2573.
13. Webb SM, Casanueva F, Wass JA. Oncological complications of excess GH in acromegaly. Pituitary. 2002; 5:21–25.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download