Abstract
There have been many advances in obesity treatment, including life-style modification and pharmacological and surgical treatments. It seems that the most remarkable advances in obesity treatment are those of pharmacological strategies. However, weight loss medications have a long history of development. The FDA has withdrawn anti-obesity drugs such as fenfluramine, dexfenfluramine, and phenylpropylamine due to unwanted side effects. Sibutramine was voluntarily withdrawn from the market, and new drugs such as rimonabant have been suspended in the middle of clinical study due to unacceptable side effects. Last year, the FDA approved two new anti-obesity drugs for the treatment of obesity. Lorcaserin is a selective 5-hydroxytryptamine receptor 2c (5-HT2c) agonist whose pharmacological mechanism of action is similar to those of fenfluramine and dexfenfluramine. However, lorcaserin is specific for 5-HT2c, which is located almost exclusively in the CNS and is not found on heart valves. Three exciting phase 3 clinical trials for lorcaserin have been published recently. Lorcaserin has been shown to successfully result in weight reduction, and the drug was not found to lead to heart disease, as is the case with some other such drugs. Furthermore, the FDA also approved controlled release phentermine/topiramate (PHEN/TPM CR), a drug composed of immediate-release phentermine and controlled-release topiramate. Weight reduction by PHEN/TPM CR is better than any other anti-obesity drugs in the world. Along with this excellent efficacy, however, come painful side effects that clinicians should consider.
Go to : 
REFERENCES
1. SClinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults-The Evidence Report. National Institutes of Health. Obes Res. 1998; 6(Suppl 2):51S–209S.
2. SBays H. Phentermine, topiramate and their combination for the treatment of adiposopathy ('sick fat') and metabolic disease. Expert Rev Cardiovasc Ther. 2010; 8:1777–801.
3. SLam DD, Garfield AS, Marston OJ, Shaw J, Heisler LK. Brain serotonin system in the coordination of food intake and body weight. Pharmacol Biochem Behav. 2010; 97:84–91.
4. SRoss EM, Roberts WC. The carcinoid syndrome: comparison of 21 necropsy subjects with carcinoid heart disease to 15 necropsy subjects without carcinoid heart disease. Am J Med. 1985; 79:339–54.
6. SThomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, Al-Shamma H, Smith B, Chalmers D, Behan D. Lorcaserin, a novel selective human 5-hydroxy-tryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther. 2008; 325:577–87.

7. SFidler MC, Sanchez M, Raether B, Weissman NJ, Smith SR, Shanahan WR, Anderson CM. BLOSSOM Clinical Trial Group. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011; 96:3067–77.
8. SO'Neil PM, Smith SR, Weissman NJ, Fidler MC, Sanchez M, Zhang J, Raether B, Anderson CM, Shanahan WR. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012; 20:1426–36.
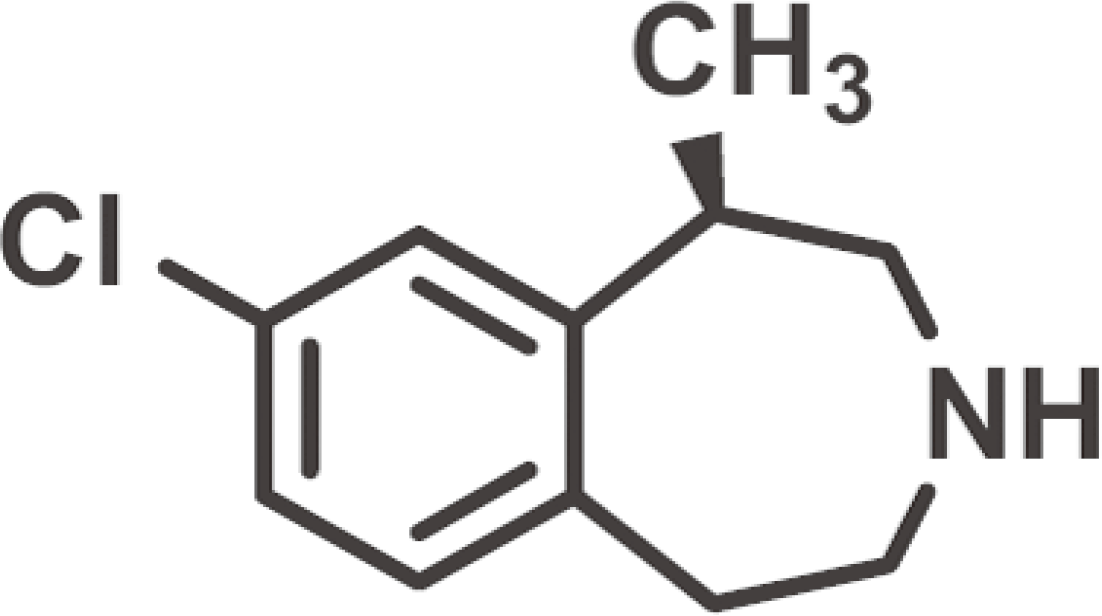
9. SSmith BM, Smith JM, Tsai JH, Schultz JA, Gilson CA, Estrada SA, Chen RR, Park DM, Prieto EB, Gallardo CS, Sengupta D, Dosa PI, Covel JA, Ren A, Webb RR, Beeley NR, Martin M, Morgan M, Espitia S, Saldana HR, Bjenning C, Whelan KT, Grottick AJ, Menzaghi F, Thomsen WJ. Discovery and structure-activity relationship of (1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine (Lorcaserin), a selective serotonin 5-HT2C receptor agonist for the treatment of obesity. J Med Chem. 2008; 51:305–13.
10. SRothman RB, Baumann MH. Appetite suppressants, cardiac valve disease and combination pharmacotherapy. Am J Ther. 2009; 16:354–64.
11. SVerrotti A, Scaparrotta A, Agostinelli S, Di Pillo S, Chiarelli F, Grosso S. Topiramate-induced weight loss: a review. Epilepsy Res. 2011; 95:189–99.
12. STremblay A, Chaput JP, Bérubé-Parent S, Prud'homme D, Leblanc C, Alméras N, Després JP. The effect of topiramate on energy balance in obese men: a 6-month double-blind randomized placebo-controlled study with a 6-month open-label extension. Eur J Clin Pharmacol. 2007; 63:123–34.

13. SGadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, Day WW. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011; 377:1341–52.
14. SGarvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, Schwiers M, Day WW, Bowden CH. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012; 95:297–308.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download