Abstract
The prevalence of obesity is steadily increasing worldwide and is commonly associated with metabolic diseases including hypertension, hyperlipidemia, and type 2 diabetes as well as increased mortality. Bariatric surgery is an effective treatment modality for patients with severe obesity and type 2 diabetes that are refractory to conventional treatments. We performed bariatric surgery (biliopancreatic diversion with duodenal switch) in a 23-year-old man with severe obesity and uncontrolled type 2 diabetes. Before surgery, the patient experienced continuous weight gain and aggravated glycemic control despite dietary restrictions, exercise, and medications including high dose insulin. After surgery, his weight was reduced by 17 kg and he was able to stop insulin treatment. This case suggests that bariatric surgery is an effective therapeutic option when severe obesity and type 2 diabetes are refractory to usual treatments.
Go to : 
REFERENCES
1. Korea Centers for Disease Control and Prevention. The forth Korea National Health and Nutrition Examination Survey (KNHANES IV-2), 2008. Seoul: Korea Centers for Disease Control and Prevention;2009.
2. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010; 303:235–41.

3. Lee SY. Current status and countermeasure of morbid obesity in Korea. Korean J Obes. 2005; 14(Suppl 1):S157–69.
4. Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr. 1992; 55(2 Suppl):S615–9.
5. Smith BR, Schauer P, Nguyen NT. Surgical approaches to the treatment of obesity: bariatric surgery. Endocrinol Metab Clin North Am. 2008; 37:943–64.

6. Lee SW, Hwang SU, Choi SH, Kim DH. A case of bariatric surgery in a patient with Prader-Willi syndrome and severe morbid obesity. J Korean Soc Pediatr Endocrinol. 2005; 10:229–35.
7. Han SM, Kim WW. The 3-year results of laparoscopic sleeve gastrectomy for the treatment of Korean morbid obesity. J Korean Surg Soc. 2007; 73:400–5.
8. Lee H, Kim M, Kwon H, Song K, Kim E. The impact of metabolic and bariatric surgery on morbidly obese patients with type 2 DM. J Korean Surg Soc. 2010; 79:8–13.

9. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995; 222:339–50. discussion 350-2.

10. Han SM, Kim WW. Early management of type 2 diabetes mellitus after sleeve gastrectomy in morbid obesity. J Korean Surg Soc. 2005; 69:304–9.
11. NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991; 115:956–61.
12. American Diabetes Association. Standards of medical care in diabetes-2010. Diabetes Care. 2010; 33(Suppl 1):S11–61.
13. Bult MJ, van Dalen T, Muller AF. Surgical treatment of obesity. Eur J Endocrinol. 2008; 158:135–45.

14. Sjostrom CD, Lissner L, Wedel H, Sjostrom L. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res. 1999; 7:477–84.
15. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004; 292:1724–37.

16. Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009; 122:248–56.

17. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346:393–403.

18. Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004; 89:2608–15.

19. Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery. 2007; 142:621–32. discussion 632-5.

20. Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LM. Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007; 357:741–52.

Go to : 
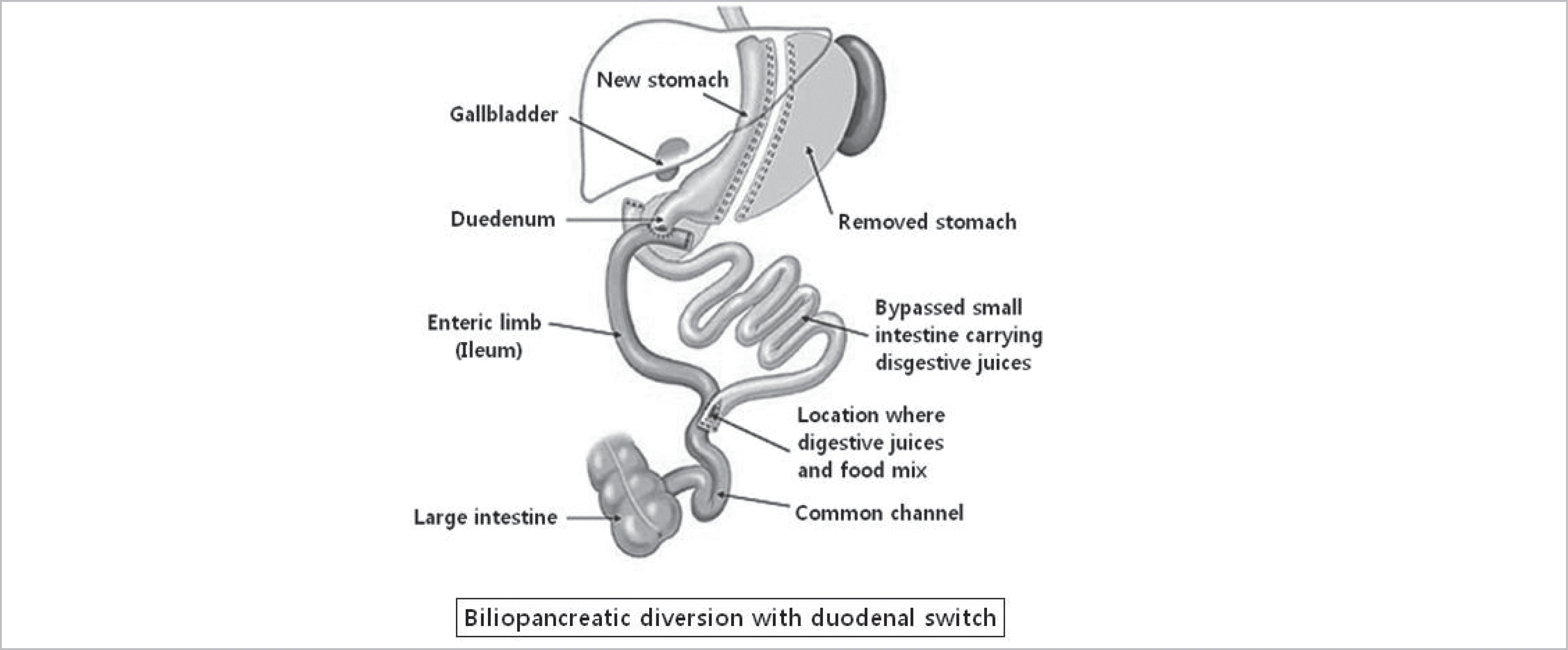 | Fig. 1.Schematic of the biliopancreatic diversion with duodenal switch procedure. Sleeve gastrectomy was performed. The duodenum was divided immediately beyond the pylorus and the alimentary limb was connected to the duodenum while the biliopancreatic limb was connected to the ileum (75 cm) proximal to the ileocecal valve. |
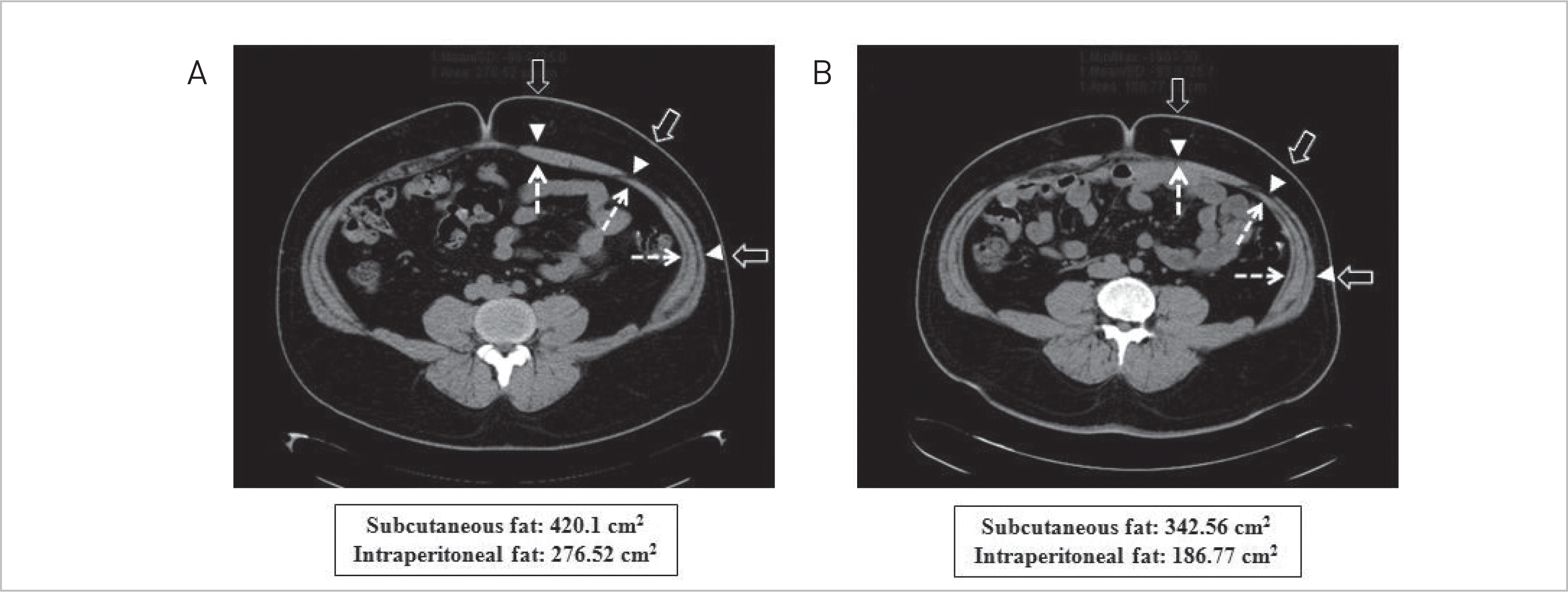 | Fig. 2.Subcutaneous fat (between open arrow and arrow head) and intraperitoneal fat (inside of dot line arrow) was measured by fat computed tomography. (A) Three months prior to operation. (B) Five months after operation. |
Table 1.
Indications for bariatric surgery for the treatment of severe obesity (adapted from NIH conference Ann Intern Med 1991;115:956-61 [11])
Table 2.
Perioperative anthropometric and biochemical changes




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download