Abstract
Central pontine and extrapontine myelinolysis are well-recognized osmotic demyelination syndromes related to the rapid correction of hyponatremia, chronic alcoholism, and malnutrition. They are reported to show brain stem signs and various movement disorders. A 58-year-old man with a history of chronic alcoholism was admitted for dysarthria, dysphagia, and gait disturbance that had developed five days after a right forearm cellulitis. Magnetic resonance imaging revealed demyelinating patterns in the central portion of the pons and both thalami. He showed severe extrapyramidal symptoms with truncal swaying and postural instability that resulted in severe gait disturbance. Postural instability showed little improvement after conventional physical therapy, but his symptoms markedly improved after five days of dopamine administration. Cessation of dopamine agents was attempted two times, but postural instability and gait disturbance recurred. Therefore, medication was continued for one year. The patient showed stable gait and no further deterioration of postural instability during dopamine therapy.
Osmotic demyelination syndrome (ODS) is a demyelinating disease that includes not only the pons (i.e., central pontine myelinolysis, CPM), but also other areas of the central nervous system, principally the thalami, globus pallidus, putamen, lateral geniculate body, and white matter of the cerebellum (i.e., extrapontine myelinolysis, EPM1). The cause and pathogenesis of ODS remain unclear, but it is often associated with rapid correction of hyponatremia, chronic alcoholism, malnutrition, prolonged diuretic use, extensive burns, and liver failure.2 Clinical symptoms and signs are inconsistent and vary from a mild tremor or dysarthria to locked-in syndrome.3 In addition, extrapyramidal symptoms, which manifest as various movement disorders such as parkinsonism, dystonia, and catatonia, can be caused by brain damage like osmotic demyelination syndrome.2,4
The diagnosis, prevention, and management of ODS are relatively well-reported, but symptomatic treatment of the various neurological deficits such as extrapyramidal syndrome has not been established. In particular, extrapyramidal syndrome without accompanying parkinsonism has rarely been reported in ODS. According to the literature, the prognosis for CPM and EPM is poor, and mortality as high as 50% has been reported.5 Limiting functional loss in these patients is crucial; however, there are no guidelines or studies that have addressed the rehabilitative strategies during the recovery phase in these patients.
In this case report, we describe a patient with chronic alcoholism with CPM and EPM accompanied by extrapyramidal syndrome who showed limited functional recovery during conventional physical therapy but had a good response after dopaminergic treatment.
A 58-year-old man with a 20-year history of more than 60 g of alcohol use per day was admitted to the local hospital with a forearm laceration and cellulitis. After five days, he suddenly developed ataxia, dysarthria, dysphagia and choreoathetosis. Local brain magnetic resonance imaging (MRI) performed two weeks after symptom onset showed signs suspicious of pons and left basal ganglia infarction. He was admitted to the department of neurosurgery for further evaluation. Laboratory evaluation performed at the time of symptom onset and upon admission showed no evidence of electrolyte imbalance or other noticeable abnormalities. His medical history was unremarkable except for a three-year history of hyperlipidemia that was being treated with medication. He had smoked 20 cigarettes a day for 30 years and drank more than a bottle of Soju (Korean gin) daily for 20 years. He also had a family history of hepatoma. His vital signs when admitted to the department of neurosurgery included a blood pressure of 90/70 mmHg, pulse rate of 80 beats/minute, respiratory rate of 20 breaths/minute, and temperature of 37.3℃. The laboratory data at that time showed normal values, including a serum sodium of 140 mEq/L (normal range: 135~145), potassium of 4.5 mEq/L (normal range: 3.5~5.5), chloride of 106 mEq/L (normal range: 98~110), aspartate aminotransferase of 16 U/L (normal range: 0~40), alanine aminotransferase of 11 U/L (normal range: 0~40), and osmolality of 281 mOsm/kg (normal range: 289~308). Glucose and erythrocyte sedimentation rate were abnormal, with a glucose of 107 mg/dl (normal range: 50~100) and an erythrocyte sedimentation rate of 30 mm/h (normal range: 0~15). On the first neurological examination, the patient was alert and well oriented (GCS 15), and his muscle powers were close to normal in all extremities. Diffusion-weighted (DWI) and T2-weighted MRI of the brain revealed high signal intensities in the central portion of the pons and both thalami. Axial and sagittal T1-weighted MRI showed low signal intensities in the central portion of the pons and both thalami (Fig. 1). These findings were compatible with ODS.
After conservative care at the neurosurgery department, he was transferred to the department of rehabilitation medicine 25 days after the onset of symptoms. Neurological examination revealed no improvement compared to the initial exam except for brisk reflexes in the lower limbs. He showed mild cognitive dysfunction (MMSE 23) and time disorientation. The initial Modified Barthel Index (MBI) was 79 points. The patient complained of a choking sensation and swallowing difficulty, and video fluoroscopic swallowing study (VFSS) showed vallecula residue and direct aspiration in liquids (dysphagia outcome severity level 1, penetration aspiration scale 8). He underwent dysphagia therapy and was put on a dysphagia diet with thicker liquids. An additional anxiolytic, buspirone (15 mg), was prescribed after the patient complained of anxiety.
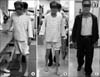
The patient had severe postural instability and truncal swaying; he could barely stand without support and was only able to walk under maximal assistance with short strides and severe truncal ataxia. For functional truncal balance evaluation, we used Berg's Balance Scale (BBS). The initial score was 10 points, indicating severe truncal imbalance. After intensive physical and dysphagia therapy, the patient showed marked improvement of dysarthria and dysphagia, but the gait disturbance remained unchanged due to severe postural instability and involuntary movement (Fig. 2A). The patient was started on dopaminergic treatment 33 days after the onset of symptoms. After five days of dopaminergic therapy, treatment with levodopa/carbidopa (100/25 mg) led to marked improvement in the patient's postural instability, truncal swaying, and ataxic gait. He could walk with minimal support without choreoathetosis or truncal ataxia. Follow-up BBS showed improvement to 42 points (Fig. 3). In order to confirm the effect of the dopaminergic agent on ODS, levodopa/carbidopa was tapered under the patient's consent. One day after drug discontinuation, the patient complained of subjective worsening of his gait pattern, and follow-up BBS showed deterioration to 32 points. Readministration and further increase of the levodopa/carbidopa dosage to 400/100 mg led to gradual improvement with a BBS of 42 points. Upon discharge, he was able to walk under supervision without any postural instability (Fig. 2B).
At the time of discharge, the MBI score was improved to 88. In addition, the patient's dysphagia showed improvement with no vallecula residue and no direct aspiration in liquids (dysphagia outcome severity level 6, penetration aspiration scale 1), and he was put on a normal diet including liquids. After discharge, he complained of mild dizziness, so we added dimenhydrinate (300 mg) and ginkgo biloba (80 mg) to his treatment regimen. Additional medication did not affect his gait function.
At one-year follow-up, he had improved to his premorbid functional state with a BBS of 56 points. We attempted to taper his medication. The dopaminergic agent levodopa/carbidopa was slowly reduced to 250/25 mg, and buspirone, dimenhydrinate, and ginkgo biloba were reduced to 10, 100, and 40 mg, respectively. While the patient showed no interval change with 500/50 mg of levodopa/carbidopa, he re-experienced truncal swaying and gait disturbance when the dosage was reduced to 250/25 mg. A follow-up BBS evaluation after the second discontinuation showed deterioration to 48 points. Thus, levodopa/carbidopa was increased to 500/50 mg, but the other medications (buspirone, dimenhydrinate, ginkgo biloba) were maintained at the same dosage. After the re-increase of the dopaminergic agent, the patient was able to maintain a stable gait and good balance (Fig. 2C).
In this report, we present a rare case of combined CPM and EPM in which the patient showed refractory recovery under conventional physical therapy but showed a good response to dopaminergic administration. A similar case of CPM and EPM associated with alcohol withdrawal has been reported,3 but there have been few reports on the management of these patients in the rehabilitation stages.
CPM and EPM, also known as ODS, are demyelinating disorders that are caused by osmotic imbalances.3 The cause and pathogenesis of ODS remain unclear, but underlying chronic disease or rapid correction of hyponatremia may be central to the development of ODS. Especially, chronic alcoholism is a common associated risk factor for developing ODS with normonatremia.6 It is believed that osmotic change due to decreased intake of food or water during heavy drinking or alcohol withdrawal may put alcoholics at a greater risk of cell shrinkage and hence demyelination.3,7
There are three possible types of myelinolysis: CPM alone, EPM alone, and CPM and EPM together.2 Although these conditions share the same pathology and time course, they differ in clinical manifestations. CPM is associated with flaccid quadriparesis, dysarthria, dysphagia, ophthalmoplegia, ataxia, and reflex changes. EPM is characterized by tremor, ataxia, gait disturbance, extrapyramidal symptoms, emotional lability, disorientation, and movement disorders, including mutism, parkinsonism, dystonia, choreoathetosis, and catatonia.1,2 Although rare, when CPM and EPM occur together, the combined symptoms suggest tragic neurological injury and may be difficult to recognize because the extrapontine manifestations are often masked by a severe pontine lesion.2,8
Dopaminergic agents have been reported to be effective in case reports of CPM or EPM.8,9 In one CPM case, the levodopa/carbidopa dosage was 125/25 mg, and medication was maintained up to seven years.8 In one EPM case, the levodopa/carbidopa dosage was 100/25 mg, and the medication was maintained for six months.9 But dopaminergic agents have not been reported to be effective in case reports of CPM and EPM presented together. Our case report is clinically relevant in that it showed the efficacy of dopaminergic agents when CPM and EPM presented together with severe gait impairment; however, more research is warranted to determine the optimal dosage and duration of dopaminergic therapy.
Our patient had both CPM and EPM involving the pons and bilateral thalami. His symptoms consisted of dysarthria, dysphagia, truncal ataxia, truncal swaying, choreoathetosis, and gait disturbance. Among these symptoms, the patient's main problems were extrapyramidal symptoms (truncal ataxia, truncal swaying, choreathetosis, and gait disturbance). Because there were no definite features of parkinsonism (i.e., resting tremor, bradykinesia, rigidity, or asymmetric onset), we used the BBS for truncal ataxia and gait disturbance evaluation instead of the more conventional Simpson-Angus Scale or Barners Akathisia Rating Scale. Although our patient's gait disturbance may have been attributable to the pontine lesion, extrapyramidal symptoms, including truncal swaying and postural instability, may have been important contributing factors.
In CPM, dopaminergic agents have been proven to be beneficial in patients with bradykinesia, rigidity, and tremor.8 In EPM, dopaminergic agents have been shown to improve bradykinesia, gait disturbance, cogwheel rigidity, and dysarthria.9 The pathogenesis of EPM with parkinsonism is believed to be due to osmotic injury causing demyelination of the nigrostriatal dopaminergic pathway, resulting in destruction of the striatal nerve terminals.2,9 Our case is clinically significant in that it is the case report that showed positive dopaminergic response in a case where CPM and EPM was involved simultaneously. It is also different from previous CPM and EPM cases8,9 in that the patient only showed extrapyramidal symptoms without any features of parkinsonism. Nonetheless, dopaminergic agents proved to be equally effective in our patient. This is probably due to the close input and output connection between the basal ganglia, thalami, and substantia nigra;10 however, further research is needed to establish the exact pathogenesis of CPM/EPM and to elucidate the exact mechanism of the good functional gains observed in our patients only during dopaminergic agent administration.
In conclusion, this is a rare case report of combined CPM and EPM in which the patient presented with extrapyramidal syndrome without parkinsonism that showed a favorable response to dopaminergic treatment. These findings suggest that physiatrists should not only pay attention to the diagnosis of CPM/EPM, but also be aware that the outcome and prognosis of these patients can be changed dramatically with the administration of dopaminergic agents during their rehabilitation stages. Dopaminergic therapy should be considered for CPM/EPM patients who show refractory recovery with conventional physical therapy during their rehabilitation stages.
Figures and Tables
Fig. 1
Diffusion-weighted (DWI) (A) and T2-weighted (B) MRI show high signal intensity in the central portion of the pons and both thalami. Axial and sagittal T1-weighted (C) MRI show low signal intensity in the central portion of the pons and both thalami.
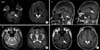
Fig. 2
(A) Patient with severe truncal ataxia. (B) Patient with improved postural stability after administration of levodopa/carbidopa (400/100 mg). (C) Patient with good recovery of mobility and balance to near premorbid levels with administration of levodopa/carbidopa (500/50 mg) at one-year follow-up in the outpatient clinic.
References
1. Martin RJ. Central pontine and extrapontine myelinolysis: the osmotic demyelination syndromes. J Neurol Neurosurg Psychiatry. 2004; 75:Suppl 3. iii22–iii28.

2. Brown WD. Osmotic demyelination disorders: central pontine and extrapontine myelinolysis. Curr Opin Neurol. 2000; 13:691–697.

3. An JY, Park SK, Han SR, Song IU. Central pontine and extrapontinemyelinolysis that developed during alcohol withdrawal, without hyponatremia, in a chronic alcoholic. Intern Med. 2010; 49:615–618.
4. Nasrallah HA, Brecher M, Paulsson B. Placebo-level incidence of extrapyramidal symptoms (EPS) with quetiapine in controlled studies of patients with bipolar mania. Bipolar Disord. 2006; 8:467–474.

5. Gocht A, Colmant HJ. Central pontine and extrapontine myelinolysis: a report of 58 cases. Clin Neuropathol. 1987; 6:262–270.
6. Kleinschmidt-Demasters BK, Rojiani AM, Filley CM. Central and extrapontine myelinolysis: then...and now. J Neuropathol Exp Neurol. 2006; 65:1–11.
8. Sadeh M, Goldhammer Y. Extrapyramidal syndrome responsive to dopaminergic treatment following recovery from central pontine myelinolysis. Eur Neurol. 1993; 33:48–50.

9. Kim JS, Lee KS, Han SR, Chung YA. Decreased striatal dopamine transporter binding in a patient with extrapontine myelinolysis. Mov Disord. 2003; 18(3):342–345.

10. Blumenfeld Hal. Neuroanatomy through clinical cases. 2nd ed. Sunderland: Sinauer;2010. p. 748–750.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download