Abstract
The purpose of this study was to investigate the changes of electromyogram activity of trunk and lower leg muscles during dynamic balance control in 20 healthy adult subjects when various experimental visual conditions were applied. Surface electromyography system was used for recording of any signals produced by muscles. Muscle activity was recorded from muscles, of which left and right sides of rectus abdominis, external obliques, longissimus thoracis, multifidus, vastus medialis, biceps femoris, gastrocnemius medialis, and tibialis anterior, and then normalized as percentage of maximum voluntary isometric contraction. All data obtained from experiment were analyzed using SPSS ver. 20.0, and two-way analysis of variance were used to determine statistical significance between two factors (3×2 factorial analysis, visual conditions vs. leg conditions). Statistical significance levels were set at α=0.05. There were significant different in biceps femoris and external obliques muscle's activities between right and left leg, showing more prominent reduction in left leg when blind vision condition was given. Significantly higher muscle activities were shown in both sides of multifidus (p<0.05), vastus medialis (p<0.001), tibialis anterior (p<0.001) and gastrocnemius medialis (p<0.001) with sighted vision and blanking vision compared to the condition of blind vision. These results confirmed that muscle activity is prominently stimulated by visual information provision, and this implies that visual input may be a major factor for maintaining of the body's balance control.
Go to : 
REFERENCES
1. Stoffregen TA, Riccio GE. An ecological theory of orientation and the vestibular system. Psychol Rev. 1988; 95:3–14.

2. Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol. 1986; 55:1369–81.

3. Nashner LM, McCollum G. The organization of human postural movements: A formal basis and experimental syn-thesis. Behav Brain Sci. 1985; 8:135–50.

4. Bae SW, Kim JI. Change the balance function with aging using computerized dynamic posturography. Exerc Sci. 2003; 12:747–56.
5. Kim YC, Jang SJ, Park MY, Park SW. Prognostic factors of ambulation in stroke patients. J Korean Acad Rehabil Med. 1992; 16:443–51.
6. Berg KO, Maki BE, Williams JI, Holliday PJ, Wood- Dauphinee SL. Clinical and laboratory measures of postural balance in an elderly population. Arch Phys Med Rehabil. 1992; 73:1073–80.
7. Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006; 35(Suppl 2):ii7-ii11.

8. Skinner HB, Barrack RL, Cook SD. Age-related decline in proprioception. Clin Orthop Relat Res. 1984; 184:208–11.

9. Congdon N, O'Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004; 122:477–85.
10. Elliott DB, Patla AE, Flanagan JG, et al. The Waterloo Vision and Mobility Study: postural control strategies in subjects with ARM. Ophthalmic Physiol Opt. 1995; 15:553–9.
11. Chen EW, Fu AS, Chan KM, Tsang WW. Balance control in very old adults with and without visual impairment. Eur J Appl Physiol. 2012; 112:1631–6.

12. Held R. Two modes of processing spatially distributed visual stimulation. Schmitt FO, editor. editor.The neurosciences: second study program. New York: Rockefeller University Press;1970. p. 317–23.
13. Lee HK, Scudds RJ. Comparison of balance in older people with and without visual impairment. Age Ageing. 2003; 32:643–9.

14. Bronstein AM. Postrography. Luxon L, Furman JM, Martini A, Stephens DG, editors. editors.Texbook of audiological medicine. clinical aspects of hearing and balance. London: CRC Press;2003. p. 747–58.
15. Nashner LM, Peters JF. Dynamic posturography in the diagnosis and management of dizziness and balance disorders. Neurol Clin. 1990; 8:331–49.

16. Chen EW, Fu AS, Tsang WW. Tai Chi training improves balance control in subjects with visual impairment. Proceeding of the Seventh Pan-Pacific Conference on Rehabilitation. 2010. Oct 23-24; Hong Kong. p. 48.
17. Kim SJ, Lee JS. The effect of multi-axis sling suspension exercise on trunk-muscle activation. Exerc Sci. 2010; 17 17:317–30.
18. De Luca CJ. Low back pain: a major problem with low priority. J Rehabil Res Dev. 1997; 34:vii–viii.
19. Kilburn KH, Thornton JC. Prediction equations for balance measured as sway speed by head tracking with eyes open and closed. Occup Environ Med. 1995; 52:544–6.

20. Winter DA. Human balance and posture control during standing and walking. Gait Posture. 1995; 3:193–214.

21. Buckley JG, Heasley KJ, Twigg P, Elliott DB. The effects of blurred vision on the mechanics of landing during stepping down by the elderly. Gait Posture. 2005; 21:65–71.

22. An SY, Kwon MJ, Kwon OY. Motor control: Theory and practical applications. 2nd ed.Seoul: Yaongmunsa;2006.
23. Horak FB, Henry SM, Shumway-Cook A. Postural pertur-bations: new insights for treatment of balance disorders. Phys Ther. 1997; 77:517–33.

24. Maki BE, McIlroy WE. The role of limb movements in maintaining upright stance: the “change-in-support” strategy. Phys Ther. 1997; 77:488–507.

25. Jacobson GP, Newman CW, Kartush JM. Handbook of balance function testing. St. Louis: Mosby;1993.
26. Day BL, Steiger MJ, Thompson PD, Marsden CD. Effect of vision and stance width on human body motion when standing: implications for afferent control of lateral sway. J Physiol. 1993; 469:479–99.

27. Seo SK, Kim SH, Kim TY. Evaluation of static balance in postural tasks and visual cue in normal subjects. J Korean Soc Phys Ther. 2009; 21:51–6.
28. Woo YK, Park JW, Choi JD, Hwang JH, Kim YH. Electromyographic activities of lower leg muscles during static balance control in normal adults. J Korean Acad Univ Trained Phys Therapists. 2004; 11:35–45.
Go to : 
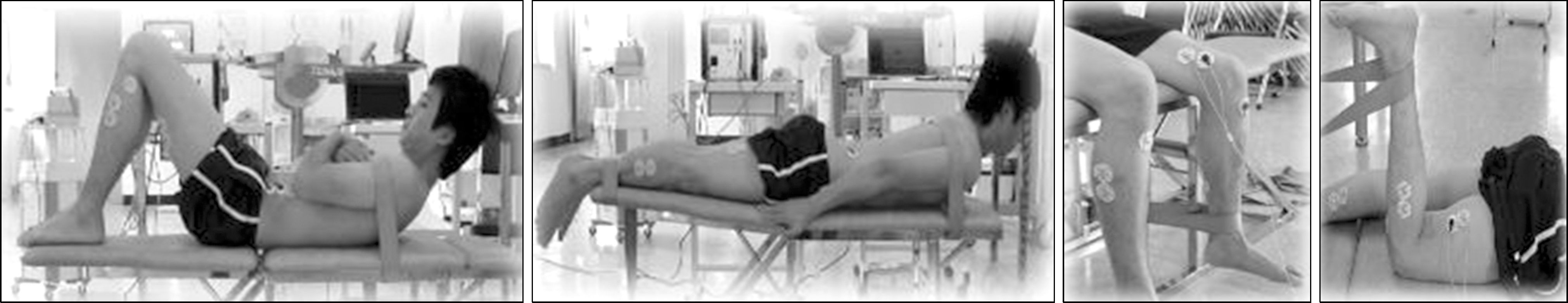
 | Fig. 1.Location of attached surface electromyogram (EMG) electrodes. (A) Rectus abdominis, External obliques. (B) Longissimus thoracis, Multifidus. (C) Vastus medialis. (D) Biceps femoris. (E) Tibialis anterior. (F) Gastrocnemius medialis. |
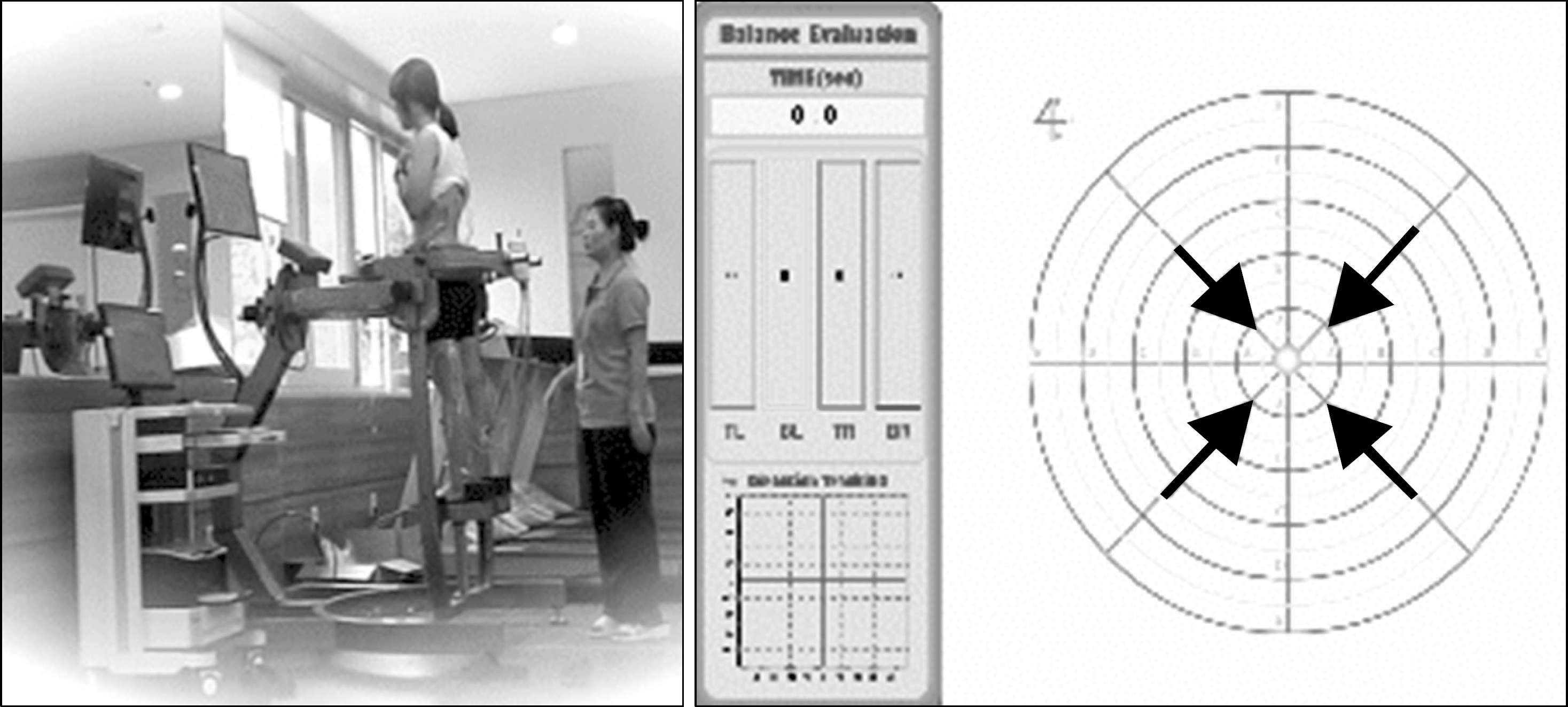 | Fig. 2.Measurement of dynamic balance using SpaceBalance three-dimensional posturography. Picture is shown the experimental condition with sighted vision used. |
Table 1.
Muscle names and location of surface electromyogram (EMG) electrodes attachment
Table 2.
Rectus abdominis and external obliques muscle activation between different vision and leg conditions measured by electromyogram (%MVIC)
| Vision condition | Main effect of leg condition | V-C | L-C | V-C vs. L-C | |||
|---|---|---|---|---|---|---|---|
| Sighted vision | Blind vision | Blanking vision | |||||
| Rectus Abdominis | 0.983 | 0.113 | 0.999 | ||||
| Leg condition | |||||||
| Right | 0.067±0.028 | 0.066±0.030 | 0.066±0.026 | 0.066±0.028 | |||
| Left | 0.057±0.030 | 0.057±0.030 | 0.056±0.028 | 0.057±0.029 | |||
| Main effect of vision condition | 0.062±0.029 | 0.061±0.030 | 0.061±0.027 | ||||
| External Obliques | 0.835 | 0.689 | 0.910 | ||||
| Leg condition | |||||||
| Right | 0.101±0.049 | 0.098±0.048 | 0.098±0.049 | 0.099±0.048 | |||
| Left | 0.100±0.053 | 0.087±0.045∗ | 0.097±0.054 | 0.095±0.050 | |||
| Main effect of vision conditions | 0.100±0.050 | 0.093±0.046 | 0.097±0.051 | ||||
Table 3.
Longissimus thorasis and multifidus muscle activation between different vision and leg conditions measured by electromyogram
| Vision condition | Main effect of leg condition | V-C | L-C V | V-C vs. L-C | |||
|---|---|---|---|---|---|---|---|
| Sighted vision | Blind vision B | Blanking vision | |||||
| Longissimus thorasis | 0.577 | 0.431 | 0.999 | ||||
| Leg condition | |||||||
| Right | 0.094±0.035 | 0.086±0.032 | 0.094±0.037 | 0.091±0.034 | |||
| Left | 0.088±0.032 | 0.081±0.035 | 0.089±0.029 | 0.086±0.032 | |||
| Main effect of vision condition | 0.091±0.033 | 0.083±0.033 | 0.091±0.033 | ||||
| Multifidus | 0.042 | 0.553 | 0.968 | ||||
| Leg condition | |||||||
| Right | 0.129±0.035∗ | 0.101±0.037 | 0.123±0.035∗ | 0.112±0.037 | |||
| Left | 0.131±0.048∗ | 0.108±0.035 | 0.129±0.051∗ | 0.123±0.045 | |||
| Main effect of vision conditions | 0.130±0.042∗ | 0.105±0.036 | 0.126±0.043∗ | ||||
Table 4.
Vastus medialis and biceps femoris muscle activation between different vision and leg conditions measured by electromyogram (%MVIC)
| Vision condition | Main effect of leg condition | V-C | L-C | V-C vs. L-C | |||
|---|---|---|---|---|---|---|---|
| Sighted vision | Blind vision | Blanking vision | |||||
| Vastus medialis | 0.001 | 0.528 | 0.950 | ||||
| Leg condition | |||||||
| Right | 0.111±0.068∗∗∗ | ∗ 0.063±0.035 | 0.105±0.057∗∗∗ | ∗ 0.093±0.058 | |||
| Left | 0.103±0.065∗∗∗ | ∗ 0.051±0.027 | 0.103±0.072∗∗∗ | ∗ 0.085±0.062 | |||
| Main effect of vision condition | 0.107±0.065∗∗ | 0.057±0.031 | 0.104±0.064∗∗ | ||||
| Biceps femoris | 0.903 | 0.734 | 0.259 | ||||
| Leg condition | |||||||
| Right | 0.067±0.038 | 0.081±0.045 | 0.073±0.045 | 0.074±0.042 | |||
| Left | 0.078±0.045 | 0.059±0.028# | 0.075±0.037 | 0.071±0.038 | |||
| Main effect of vision conditions | 0.072±0.041 | 0.070±0.039 | 0.074±0.040 | ||||
Table 5.
Gastrocnemius medialis and tibialis anterior muscle activation between different vision and leg conditions measured by electromyogram (EMG) (%MVIC)
| Vision condition | Main effect of leg condition | V-C | L-C | V-C vs. L-C | |||
|---|---|---|---|---|---|---|---|
| Sighted vision | Blind vision | Blanking vision | |||||
| Gastrocnemius medialis | 0.000 | 0.712 | 0.843 | ||||
| Leg condition | |||||||
| Right | 0.177±0.083∗∗∗ | 0.078±0.036 | 0.147±0.086∗∗ | 0.134±0.082 | |||
| Left | 0.182±0.131∗∗∗ | 0.073±0.037 | 0.169±0.137∗∗∗ | 0.141±0.119 | |||
| Main effect of vision condition | 0.180±0.107∗∗∗ | 0.075±0.036 | 0.158±0.113∗∗∗ | ||||
| Tibialis anterior | 0.000 | 0.911 | 0.851 | ||||
| Leg condition | |||||||
| Right | 0.167±0.106∗∗∗ | 0.048±0.047 | 0.149±0.074∗∗ | 0.121±0.094 | |||
| Left | 0.169±0.102∗∗∗ | 0.056±0.058 | 0.133±0.103∗∗ | 0.119±0.099 | |||
| Main effect of vision conditions | 0.168±0.102∗∗∗ | 0.052±0.052 | 0.141±0.089∗∗ | ||||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download