Abstract
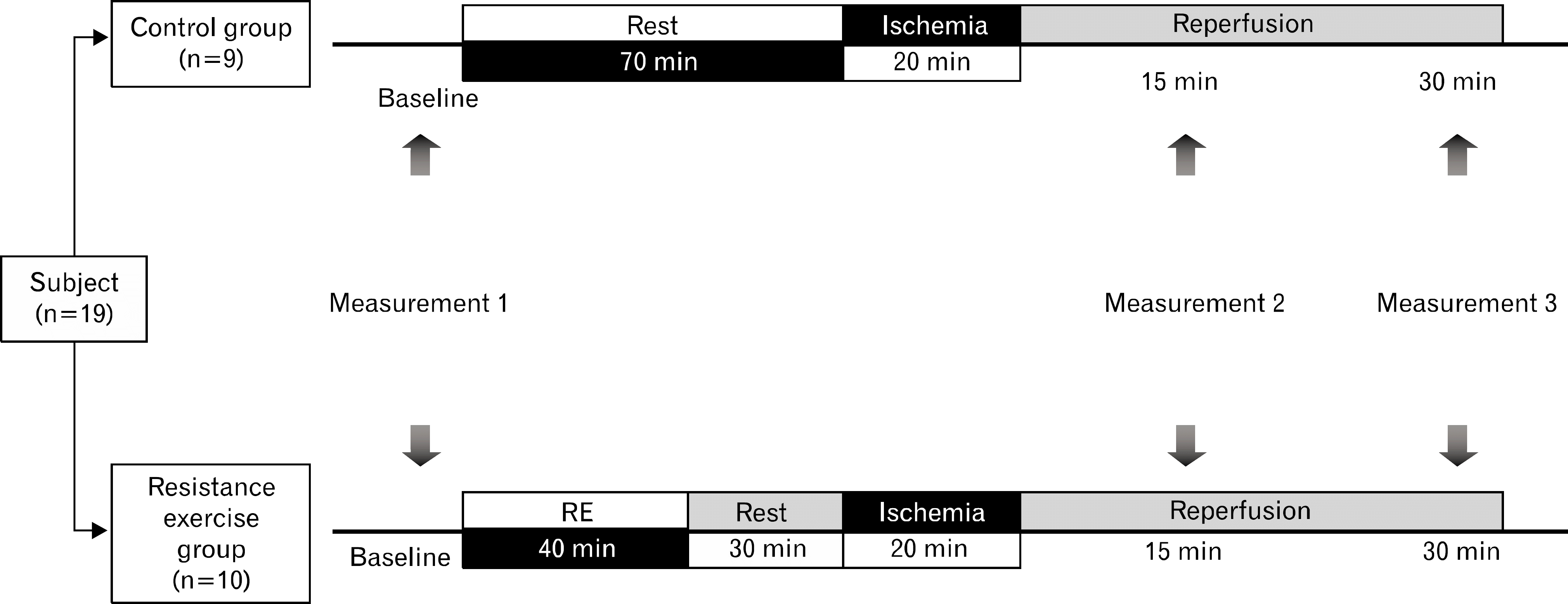
Ischemia reperfusion injury (IRI) leads to a temporary decrease in macrovascular function, but whether IRI causes microvascular dysfunction is not known. Resistance exercise involves muscular contractions that can make downstream tissues ischemic and may ischemic preconditioning the vasculature against endothelial IRI. We tested the hypothesis that an acute resistance exercise prior to IRI would prevent or attenuate IRI induced macro- and microvascular dysfunction in healthy young adults. Nineteen healthy young subjects (age 22±2 years) were randomly assigned to either a resistance exercise group (n=10) as a model to produce ischemic preconditioning or a control group (n=9). The resistance exercise was performed eight types of systemic resistance exercise. Ischemia was induced by inflating a cuff placed around the upper arm to 200 mm Hg for 20 minutes. carotid-femoral pulse wave velocity (cfPWV) as index of macrovascular function and reactive hyperemia index (RHI) using by fingertip arterial tonometry as index of microvascular function were measured at baselines and 15 and 30 minutes after ischemia reperfusion injury. cfPWV was increased in control group but decreased in resistance exercise group following IRI. There was a significant interaction effect between resistance exercise group and control group for cfPWV (p=0.022). The RHI was unaffected following IRI and also unchanged by a resistance exercise. These findings show that ischemia reperfusion caused macrovascular dysfunction but not microvascular dysfunction. However, this macrovascular dysfunction following IRI was not shown in the resistance exercise group. Thus, an acute bout of resistance exercise prior to ischemia may prevent against ischemia reperfusion injury induced macrovascular dysfunction.
Go to : 
References
2. Gori T, Lisi M, Forconi S. Ischemia and reperfusion: the endothelial perspective: a radical view. Clin Hemorheol Microcirc. 2006; 35:31–4.
3. Kharbanda RK, Peters M, Walton B, et al. Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia-reperfusion in humans in vivo. Circulation. 2001; 103:1624–30.

4. Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol. 2005; 46:450–6.
5. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and metaanalysis. J Am Coll Cardiol. 2010; 55:1318–27.
6. Cohn JN, Duprez DA, Grandits GA. Arterial elasticity as part of a comprehensive assessment of cardiovascular risk and drug treatment. Hypertension. 2005; 46:217–20.

7. Alhejily W, Aleksi A, Martin BJ, Anderson TJ. The effect of ischemia-reperfusion injury on measures of vascular function. Clin Hemorheol Microcirc. 2013 May 29. [Epub].http://dx.doi.org/10.3233/CH-131741.

8. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986; 74:1124–36.

9. Romanque P, Diaz A, Tapia G, Uribe-Echevarria S, Videla LA, Fernandez V. Delayed ischemic preconditioning protects against liver ischemia-reperfusion injury in vivo. Transplant Proc. 2010; 42:1569–75.

10. Frasier CR, Moore RL, Brown DA. Exercise-induced cardiac preconditioning: how exercise protects your achy-breaky heart. J Appl Physiol (1985). 2011; 111:905–15.

11. Lee Y, Min K, Talbert EE, et al. Exercise protects cardiac mitochondria against ischemia-reperfusion injury. Med Sci Sports Exerc. 2012; 44:397–405.

12. DeVan AE, Umpierre D, Lin HF, et al. Habitual resistance exercise and endothelial ischemia-reperfusion injury in young adults. Atherosclerosis. 2011; 219:191–3.

13. Baechle TR, Earle RW. National Strength & Conditioning Association (U.S.). Essentials of strength training and conditioning. 2nd ed.Champaign, IL: Human Kinetics;2000.
14. DeVan AE, Anton MM, Cook JN, Neidre DB, Cortez-Cooper MY, Tanaka H. Acute effects of resistance exercise on arterial compliance. J Appl Physiol. 2005; 98:2287–91.

15. Shantsila E, Wrigley B, Shantsila A, et al. Ethnic differences in macrovascular and microvascular function in systolic heart failure. Circ Heart Fail. 2011; 4:754–62.

16. Van Bortel LM, Duprez D, Starmans-Kool MJ, et al. Clinical applications of arterial stiffness, Task Force III: recommendations for user procedures. Am J Hypertens. 2002; 15:445–52.

17. Chiariello M, Ambrosio G. Myocardial damage during ischaemia and reperfusion. Rev Port Cardiol. 1994; 13:655–9.
18. Onegbu N, Kamran H, Sharma B, et al. The effects of ischemia with and without remote conditioning on hyperemia induced decline in carotid-radial pulse wave velocity. Atherosclerosis. 2012; 220:151–4.

19. Vlachopoulos C, Dima I, Aznaouridis K, et al. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005; 112:2193–200.

20. Gori T, Di Stolfo G, Sicuro S, Dragoni S, Parker JD, Forconi S. The effect of ischemia and reperfusion on microvascular function: a human in vivo comparative study with conduit arteries. Clin Hemorheol Microcirc. 2006; 35:169–73.
21. Luksha L, Agewall S, Kublickiene K. Endothelium-derived hyperpolarizing factor in vascular physiology and cardiovascular disease. Atherosclerosis. 2009; 202:330–44.

22. Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res. 2003; 92:e31–40.

23. Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000; 86:494–501.
24. Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000; 190:255–66.

25. Chaves EA, Pereira-Junior PP, Fortunato RS, et al. Nandrolone decanoate impairs exercise-induced cardioprotection: role of antioxidant enzymes. J Steroid Biochem Mol Biol. 2006; 99:223–30.

26. Devan AE, Umpierre D, Harrison ML, et al. Endothelial ischemia-reperfusion injury in humans: association with age and habitual exercise. Am J Physiol Heart Circ Physiol. 2011; 300:H813–9.

27. Soufi FG, Saber MM, Ghiassie R, Alipour M. Role of 12-week resistance training in preserving the heart against ischemia-reperfusion-induced injury. Cardiol J. 2011; 18:140–5.
Go to : 
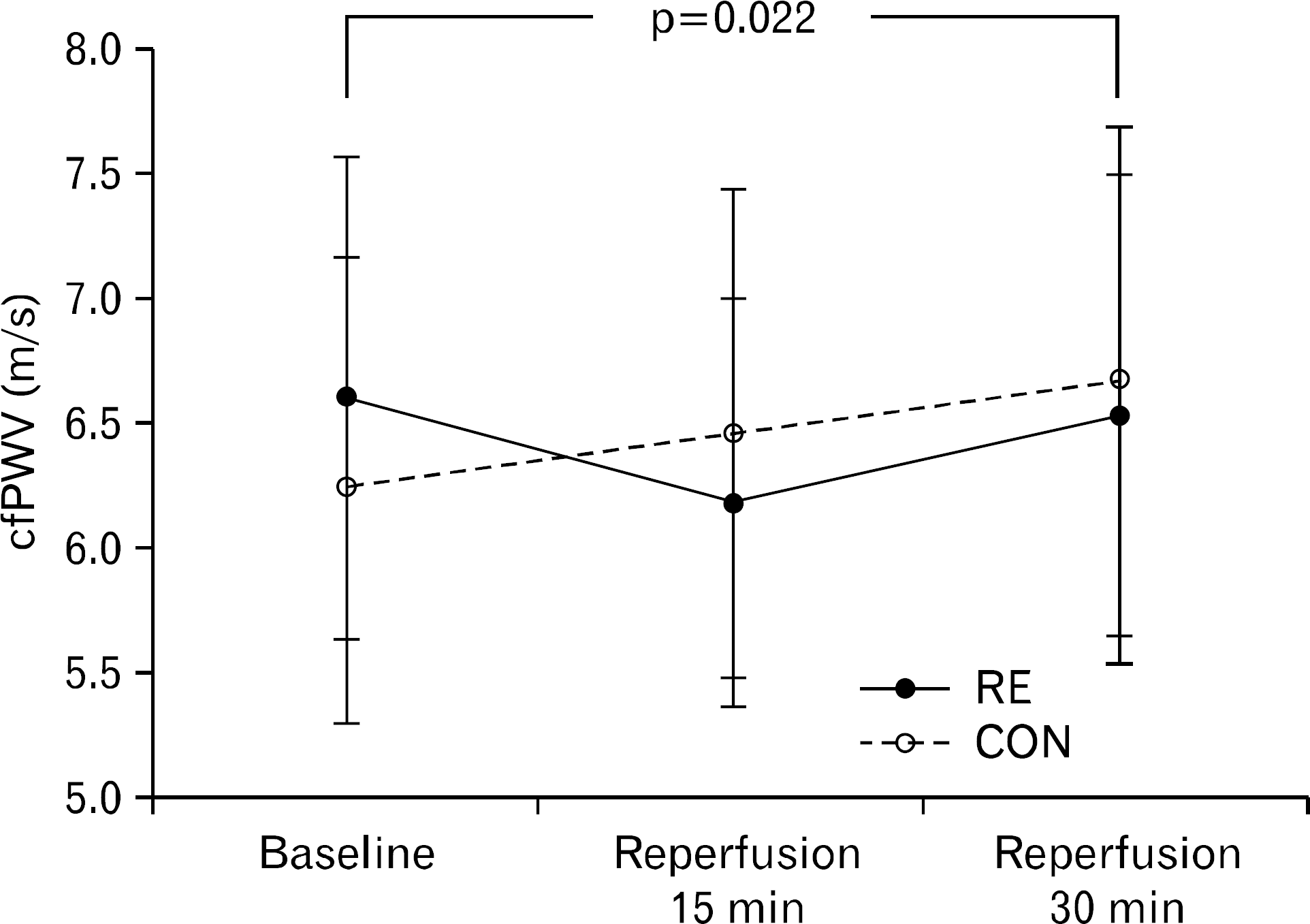 | Fig. 3.Carotid femoral pulse wave velocity response to is-chemia-reperfusion injury. Values are presented as mean± standard deviation. p-value for time and trial interaction effect. RE: resistance exercise group, CON: control group, cfPWV; carotid femoral pulse wave velocity. |
 | Fig. 4.RHI response to ischemia-reperfusion injury. Values are presented as mean±standard deviation. RE: resistance exercise group, CON: control group, RHI: reactive hyperemia index, NS: not significant, p-value for time and trial interaction effect. |
Table 1.
Physical characteristics of the subjects
Table 2.
Augmentation index and blood pressure in response to a ischemia-reperfusion injury in a resistance exercise group and control group
| Characteristic | Baseline | Reperfusion | p-values | |
|---|---|---|---|---|
| 15 min | 30 min | |||
| AIx@75 bpm (%) | 0.895* | |||
| RE | –8.45±12.62 | –8.79±7.50 | –10.20±8.48 | 0.104† |
| CON | –16.56±6.34 | –16.61±7.23 | –13.61±11.56 | 0.338‡ |
| SBP (mm Hg) | 0.992* | |||
| RE | 111.80±13.63 | 109.30±11.16 | 111.20±15.29 | 0.962† |
| CON | 109.56±13.78 | 111.67±7.95 | 110.33±10.89 | 0.579‡ |
| DBP (mm Hg) | 0.001* | |||
| RE | 64.90±7.08 | 54.40±7.06 | 58.90±6.38 | 0.281† |
| CON | 66.11±10.35 | 59.89±9.20 | 62.67±6.44 | 0.387‡ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download