Abstract
PURPOSE
This laboratory study aimed to investigate the effect of doping an acrylic denture base resin material with nanoparticles of ZnO, CaO, and TiO2 on biofilm formation.
MATERIALS AND METHODS
Standardized specimens of a commercially available cold-curing acrylic denture base resin material were doped with 0.1, 0.2, 0.4, or 0.8 wt% commercially available ZnO, CaO, and TiO2 nanopowder. Energy dispersive X-ray spectroscopy (EDX) was used to identify the availability of the nanoparticles on the surface of the modified specimens. Surface roughness was determined by employing a profilometric approach; biofilm formation was simulated using a monospecies Candida albicans biofilm model and a multispecies biofilm model including C. albicans, Actinomyces naeslundii, and Streptococcus gordonii. Relative viable biomass was determined after 20 hours and 44 hours using a MTT-based approach.
RESULTS
No statistically significant disparities were identified among the various materials regarding surface roughness and relative viable biomass.
CONCLUSION
The results indicate that doping denture base resin materials with commercially available ZnO, CaO, or TiO2 nanopowders do not inhibit biofilm formation on their surface. Further studies might address the impact of varying particle sizes as well as increasing the fraction of nanoparticles mixed into the acrylic resin matrix.
In 2016, the latest national German survey on oral health issues revealed that about 33% of senior citizens wear removable denture prostheses,1 which indicates that removable prostheses are still a frequently employed treatment option in contemporary prosthetic dentistry. However, with an estimated prevalence of 70%,2 the prevalence of denture stomatitis in denture wearers is high. Candida albicans is regarded as the most relevant causative agent in denture stomatitis,3 which regularly affects geriatric patients wearing removable prostheses that are only irregularly subject to oral hygiene. Thus, approaches that inhibit the adherence and proliferation of Candida albicans biofilms on denture base materials are necessary. Several studies have identified that metal oxides such as MgO, ZnO, or TiO2 feature antimicrobial activity against bacteria or fungi.456 In addition, recent studies highlighted that metal oxide nanoparticles such as ZnO have excellent antimicrobial properties,7 which has been attributed to the production of reactive oxygen species as well as the enrichment of nanoparticles in the cytoplasm or on the outer bacterial cell membranes.8 This laboratory study aimed to investigate the effect of blending Zno, CaO, and TiO2 nanoparticles into conventional acrylic denture base resins on Candida albicans biofilm formation. The study hypothesis was the addition of metallic oxides nanoparticles inhibits microbial proliferation on the surface of the modified denture base resins.
Standardized specimens with a diameter of 7 mm and a height of 1.5 mm were prepared by mixing 5 g powder polymer and 3 mL liquid monomer from an auto-curing acrylic denture base resin (Palapress vario, Heraeus Kulzer GmbH, Hanau, Germany). For preparation of specimens from nanoparticle-doped acrylic denture base resin, 5 g PMMA powder were supplemented with either 0.1, 0.2, 0.4, or 0.8 wt % commercially available ZnO (< 100 nm particle size, Sigma-Aldrich Co., St. Louis, MO, USA), CaO (< 160 nm particle size, Sigma-Aldrich Co.), or TiO2 (average particle size 21 nm, Sigma-Aldrich Co.) nanopowder and subjected to vigorous mixing using an overhead shaker for 30 min. All specimens were polymerized at a temperature of 55℃ and a pressure of 2 bars and were subsequently polished to high gloss using 1000/4000-grit silicon carbide grinding paper (Buehler, Lake Bluff, IL, USA) and a polishing machine (Motopol 8; Buehler, Düsseldorf, Germany). Finally, the specimens were stored in distilled water for 7 d prior to conducting the experiments to decrease the impact of residual monomers on microbial proliferation.
Using a profilometric contact surface measurement device (Perthen S6P, Feinprüf-Perthen, Göttingen, Germany) with a cut off level of 0.25, arithmetic mean deviation of their individual surface roughness profile (Ra) was measured at three randomly selected spots on each specimen. A distance of 1.75 mm was measured in one single line scan perpendicular to the grinding grooves using a standard diamond tip with a tip radius 2 µm and a tip angle of 90°. Energy dispersive x-ray spectroscopy (EDX) was qualitatively employed to identify the fractions of Zn, Ca, and Ti on the surface of the various modified denture base resin specimens.
Subsequent to collection of whole saliva by expectoration from healthy volunteer donors, the saliva was pooled, frozen, and thawed and filtrated with single-use filtration devices (Corning Bottle Top Vacuum Filter, Corning Inc., Corning, NY, USA) prior to the experiments. Candida albicans ATCC 10231, as well as Streptococcus gordonii DL1 and Actinomyces naeslundii T14V (both kindly provided by Paul Kolenbrander, NIH, Bethesda, MD, USA), were cultured separately overnight in Schaedler's broth; the growth curves were analyzed to warrant that each microorganism was in its stationary growth phase. Each microbial suspension was centrifuged (3.000 rpm, 18℃, 5 minutes); subsequently, the cell pellet was resuspended in PBS. Using a spectrophotometer (Genesys 10 UV, Thermo Spectronic, Rochester, NY, USA), an optical density of 0.3 at 540 nm was adjusted, which corresponded to a final concentration of 1.4 × 107 cells/mL (C. albicans), 2.8 × 108 cells/mL (A. naeslundii), and 2.6 × 108 cells/mL (S. gordonii).
Simulation of biofilm formation was initiated by incubating each specimen in well cell clusters with 1 mL of whole saliva for 2 hours and subsequently with 1 mL of Candida albicans ATCC 10231 suspension (monospecies biofilm formation; 15 specimens/group) or a suspension of Candida albicans ATCC 10231, Actinomyces naeslundii T14V, and Streptococcus gordonii DL1 for 2.5 hours (multispecies biofilm formation; 15 specimens/group). After 2.5 hours, microbial suspensions were carefully removed and microbial proliferation was simulated for 20 hours and 44 hours by incubation with medium. Relative viable biomass was subsequently analyzed using a MTT-based approach as described in detail previously.9
Means and standard deviations were calculated; statistical analysis was performed as a function of resin modification and biofilm formation time using two-way analysis of variance (ANOVA) guided by post-hoc analyses (Tukey) where appropriate. The level of significance (α) was set to 0.05.
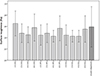
Ra values ranged between 0.04 and 0.07 µm (Fig. 1) and no significant differences were identified among the various modified denture base resins and the unmodified control (P = .093). Corresponding to the increased fractions of ZnO, CaO, and TiO2 blended into the various modified denture base resins, EDX analyses proved a slight yet continuous increase in the fractions of Zn, Ca, and Ti on the surface of the various modified denture base resin specimens (data not presented). For single-species biofilms (Fig. 2), significantly lower relative absorbance values indicating fewer viable adherent cells were identified after 44 hours than after 20 hours of biofilm formation (P = .014), whereas for multispecies biofilms (Fig. 3), absorbance values were significantly higher after 44 h than after 20 hours of biofilm formation (P < .001). Although significant differences in relative absorbance values were identified for biofilms on the specimens produced from the different modified denture base acrylic resins, no significant impact of the addition of ZnO, CaO, and TiO2 nanoparticles on both mono- and multispecies biofilm formation was identified in comparison to unmodified acrylic resin (P > .05).
The results of the present laboratory study rejected the research hypothesis, as no significant impact of the addition of ZnO, CaO, and TiO2 nanoparticles on single- and multispecies biofilm formation could be identified in this laboratory study.
The present study employed a laboratory approach to simulate biofilm formation including salivary pellicle formation and employed co-incubation of several microorganisms to adequately simulate the bioadhesive processes in the oral cavity. Nevertheless, the authors are aware that not all processes during biofilm formation can be adequately simulated with a laboratory approach; thus, the results of this study should be corroborated with a clinical study.
While the antimicrobial effects of metal oxides have frequently been addressed, limited evidence is currently available regarding the effect of doping denture base materials with metal oxides on fungal biofilm formation. With regard to their antifungal activity, Sawai and his co-workers identified a significantly more pronounced effect of MgO and CaO nanoparticles than for ZnO5; other researchers showed that coating acrylic dentures with TiO2 inhibits adhesion of Candida albicans.6
In the current study, blending ZnO, CaO, and TiO2 nanoparticles into a conventional acrylic auto-curing denture base resin produced no significant effect on relative viable biomass in both biofilm models. With regard to this aspect, the antimicrobial activity of metal oxide nanoparticles is dependent on their concentration.8 As a maximum of 0.8 wt% of each single nanopowder was blended into the conventional resin in order to prevent an impairment of the mechanical properties in this study, it might be assumed that the volumes blended into the acrylic matrix were too low to feature a relevant effect on biofilm formation. A recently published study from another group reported a significant antifungal effect of polymethyl methacrylate doped with ZnO nanoparticles on Candida albicans biofilms, observing a continuously enhanced antifungal effect with increasing ZnO concentration from 2.5 to 7.5 mass%.10 However, in contrast to the Cierech's study, the present study included simulation of salivary pellicle formation; it might be possible that the layer of salivary proteins inhibited the antimicrobial effect of the nanoparticles. Moreover, it has been reported that the antimicrobial effect of nanoparticles is size-dependent,11 suggesting that the size of the nanoparticles employed in the present study was too large for producing an antimicrobial effect. However, Raghupathi and his co-workers identified a threshold for ZnO nanoparticles at 100 nm, observing that larger nanoparticles have no bacteriostatic effect against methicillin sensitive Staphylococcus aureus strain8. While the size of the ZnO and TiO2 particles employed in the present study was lower than this threshold, it is doubtful whether these considerations can serve as a valid explanation for the results observed in this study. Nevertheless, it cannot be excluded that clustering and exsolution effects inhibited the antimicrobial activity of the nanoparticles blended into the acrylic denture base resin material. In addition to that, it might be possible that the method employed in the present study to supplement the conventional denture base resin with nanoparticles produced specimens with nanoparticles embedded into the resin matrix rather than specimens with nanoparticles that are available on their surface, which might serve as an explanation for the little effect on biofilm formation that had been observed in this study. While it might be even more effective to coat denture base resins with nanoparticles, the authors attempted to simulate a simple process to modify denture base resins with nanoparticles that could potentially be applied in a conventional dental laboratory. In addition to that, qualitative EDX analyses identified a slight yet continuous increase in Zn, Ca, and Ti fractions on the surfaces of the various denture base resins supplemented with increasing fractions of ZnO, CaO, and TiO2, which proved that the nanoparticles were also available on the surface of the modified specimens.
Within the limitations of a laboratory study, the results indicate that doping acrylic denture base resins with a maximum of 0.8 wt% ZnO, CaO, or TiO2 nanoparticles are not effective in inhibiting biofilm formation. Further studies might, however, address the impact of varying size and concentration of nanoparticles on biofilm formation.
Figures and Tables
Fig. 1
Surface roughness (Ra) of the various modified denture base resins and the unmodified control. Means and standard deviations are indicated.
References
1. Nitschke I, Stark H. Krankheits- und Versorgungspravalenzen bei jungeren Senioren (65- bis 74-Jahrige). In : Jordan AR, Micheelis W, editors. Funfte Deutsche Mundgesundheitsstudie. Cologne: Deutscher Zahnarzte Verlag DAV;2016. p. 416–451.
2. Gendreau L, Loewy ZG. Epidemiology and etiology of denture stomatitis. J Prosthodont. 2011; 20:251–260.
3. Budtz-Jorgensen E, Bertram U. Denture stomatitis I The etiology in relation to trauma and infection. Acta Odontol Scand. 1970; 28:71–92.
4. Sawai J, Igarashi H, Hashimoto A, Kokugan T, Shimizu M. Evaluation of growth inhibitory effect of ceramics powder slurry on bacteria by conductance method. J Chem Eng Jpn. 1995; 28:288–293.
5. Sawai J, Yoshikawa T. Quantitative evaluation of antifungal activity of metallic oxide powders (MgO, CaO and ZnO) by an indirect conductimetric assay. J Appl Microbiol. 2004; 96:803–809.
6. Arai T, Ueda T, Sugiyama T, Sakurai K. Inhibiting microbial adhesion to denture base acrylic resin by titanium dioxide coating. J Oral Rehabil. 2009; 36:902–908.
7. Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, Memic A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomedicine. 2012; 7:6003–6009.
8. Raghupathi KR, Koodali RT, Manna AC. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir. 2011; 27:4020–4028.
9. Susewind S, Lang R, Hahnel S. Biofilm formation and Candida albicans morphology on the surface of denture base materials. Mycoses. 2015; 58:719–727.
10. Cierech M, Kolenda A, Grudniak AM, Wojnarowicz J, Woźniak B, Gołaś M, Swoboda-Kopeć E, Łojkowski W, Mierzwińska-Nastalska E. Significance of polymethylmethacrylate (PMMA) modification by zinc oxide nanoparticles for fungal biofilm formation. Int J Pharm. 2016; 510:323–335.
11. Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C Mater Biol Appl. 2014; 44:278–284.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download