Abstract
PURPOSE
The goal of this study was to evaluate the fracture resistances of various monolithic crowns fabricated by computer-aided design and computer-aided manufacturing (CAD/CAM) with different thickness.
MATERIALS AND METHODS
Test dies were fabricated as mandibular molar forms with occlusal reductions using CAD/CAM. With different occlusal thickness (1.0 or 1.5 mm), a polymer-infiltrated ceramic network (Enamic, EN), and zirconia-reinforced lithium silicate (Suprinity, SU and Celtra-Duo, CD) were used to fabricate molar crowns. Lithium disilicate (e.max CAD, EM) crowns (occlusal: 1.5 mm) were fabricated as control. Seventy crowns (n=10 per group) were bonded to abutments and stored in water for 24 hours. A universal testing machine was used to apply load to crown until fracture. The fractured specimens were examined with a scanning electron microscopy.
RESULTS
The type of ceramics and the occlusal thickness showed a significant interaction. With a recommended thickness (1.5 mm), the SU revealed the mean load similar to the EM, higher compared with those of the EN and CD. The fracture loads in a reduced thickness (1.0 mm) were similar among the SU, CD, and EN. The mean fracture load of the SU and CD enhanced significantly when the occlusal thickness increased, whereas that of the EN did not.
CONCLUSION
The fracture loads of monolithic crowns were differently influenced by the changes in occlusal thickness, depending on the type of ceramics. Within the limitations of this study, all the tested crowns withstood the physiological masticatory loads both at the recommended and reduced occlusal
thickness.
Since ceramics were first used in clinical dentistry as restorative materials, their range of applicability has expanded from anterior to posterior teeth due to the excellent aesthetics, biocompatibility, and enhanced physical properties.1 Many types of ceramics have been gradually used in posterior fixed restorations and their survival rates are comparable to those of conventional restorative materials of single restorations in short-term observation studies.234 Posterior restorations with high-strength ceramics, such as lithium disilicate or yttria-stabilized zirconia, is clinically applicable.35 A five-year follow-up study reported that zirconia-based threeunit posterior fixed dental prostheses functioned well inside the oral cavity.6 A systematic review of the clinical outcomes of lithium disilicate-based fixed restorations also reported high short-term survival rates for single crowns.7
Even with remarkable advances in the properties of dental ceramics, clinical failures and limitations of allceramic restorations still have been reported in many studies.389 One of the most common complications is the chipping and delamination of the veneering porcelain.2 This occurs more often in posterior teeth than in anterior teeth and in molars than in premolars.1 Subsequently, experimental studies on monolithic ceramics without veneers were conducted in order to prevent chipping and fractures.10 To improve aesthetics and mechanical properties of monolithic ceramics, recent studies are mainly focused on the light transmittance, tooth color reproduction, and manufacturing conditions.11 Monolithic all-ceramic crowns are fabricated by computer-aided design and computer-aided manufacturing (CAD/CAM), which are suitable for aesthetic posterior restorations with low mechanical complication, high productivity, and the absence of veneer chipping.1213 Both lithium disilicate and zirconia-based posterior restorations exhibit favorable clinical outcomes.14
Various block-shaped ceramics are used in CAD/CAM fabrication of aesthetic restorations.15 Because these materials have different compositions and microstructures, the fracture resistance, wear, adhesiveness, durability, damage tolerance, optical properties, and machinability must be considered before restorative treatments.15 Recently, a polymerinfiltrated ceramic network (PICN), in which polymers are infiltrated into a porous ceramic network, has been developed in order to mimic the physical properties of natural tooth and to overcome the brittleness of ceramics causing wear on antagonistic tooth.16 The PICN comprises interpenetrating phases of feldspathic ceramic and methacrylate polymers; it exhibits an elastic modulus similar to that of human dentin and has higher flexural strength compared to feldspathic ceramics.1617 It shows good hardness and damage tolerance because the two interconnected phases limit the progression of cracking.16 It also has outstanding machinability because of its low brittleness, flexibility, and high fracture resistance.17 When an external force is exerted on the surface of the PICN, its plastic deformation and creep may absorb the energy.1718192021 A zirconia-containing lithium silicate (ZLS) was also recently developed as a material amenable to CAD/CAM fabrication.11 Upon crystallization, which occurs with the addition of 10 wt% of zirconium oxide as a nucleating agent to a lithium-disilicate-based multicomponent glass ceramic, the material acquires a fine and uniform microstructure with an average grain size of 0.5 – 0.7 µm. According to the manufacturer, the ZLS can be used for fabricating various fixed dental prostheses for anterior and posterior teeth, since it has optical and physical properties comparable to those of lithium disilicate.
To withstand masticatory forces, many studies have reported that the fracture resistance of ceramics used in posterior restorations determines treatment success.222324 Each manufacturer provides guidelines on the tooth reduction and thickness of the materials used for posterior crowns. For the lithium disilicate ceramics, a deep chamfer margin of 1 mm, as well as a minimum reduction of 1.5 mm on the occlusal surfaces is recommended for the posterior restoration. However, when the interocclusal space is limited or the tooth structure remains little due to extensive caries, it is often difficult to reduce the occlusal or axial surfaces of the abutment as the recommendation of the manufacturer. If the materials show sufficient fracture strength even in reduced thickness, they could be applicable in various clinical situations.
The purpose of the present study was to analyze the fracture resistances of molar-form monolithic ceramic crowns according to their thicknesses using CAD/CAM technology. The null hypothesis of the present study was the following: the fracture loads of the CAD/CAM-fabricated monolithic crowns are the same regardless of the type of ceramics and the amount of occlusal thickness.
A half arch of the mandibular arch dentiform (D51DP-500A; Nissin Dental Products, Kyoto, Japan), excluding the first molar, was scanned using an intraoral digital scanner (TRIOS Pod Color; 3Shape, Copenhagen, Denmark), and a CAD design of the first molar was generated with reductions in the occlusal and axial surfaces (3Shape Model Builder; 3Shape). The circular reduction at the cervical margin was set to produce a 1-mm-wide chamfer finish line, the convergence angle was set to 6°, and the occlusal anatomical reduction of 1.5 mm was conducted in a multi-planar manner (Fig. 1). The ideal anatomical tooth form in the CAD software library (Smile Composer; 3Shape) was used as the reference and the occlusal thicknesses of the molar-shaped definitive crowns were first set to 1.5 mm (recommended condition) and then changed to 1.0 mm (reduced condition) (Fig. 1). The cross-sectional thicknesses of specific five areas (mesiobuccal cusp, distobuccal cusp, mesio-occlusal fossa, distal fossa, and central fossa) were measured using the CAD software to confirm the reduction of the occlusal surface and the thicknesses of the restoration. Each of the cement layer thickness was set to 50 µm. The lower part of the test abutment die was designed in a pillar shape by using a commercial CAD program (PowerSHAPE; Delcam, Birmingham, UK) to simplify further laboratory testing.
The manufacturers' information of the monolithic ceramics used in this study was shown in Table 1. The design data of the CAD-based molar crowns were used to establish seven experimental groups with two different occlusal thicknesses: 1.5 mm (recommended condition) and 1.0 mm (reduced condition). Three different CAD/CAM ceramic blocks, including the PICN (Enamic [EN]; VITA Zahnfabrik, Bad Säckingen, Germany) and two types of zirconia-reinforced lithium silicate (Suprinity [SU]; VITA Zahnfabrik, Celtra Duo [CD]; Dentsply DeTrey, Konstanz, Germany) were fabricated into molar-shaped crowns by using a five-axis milling machine (Trione G; Dio, Busan, Korea). As a control group, the lithium disilicate (e.max CAD [EM]; Ivoclar Vivadent, Schaan, Liechtenstein) molar crowns (occlusal thickness: 1.5 mm only) were also made with the same milling machine. Subsequently, a total of 70 monolithic ceramic crowns (n = 10 per group) were prepared. Crystallization for the SU crown and glazing process required for each ceramic material after the milling were performed in a furnace (Programat P310; Ivoclar Vivadent) according to the corresponding manufacturer's protocols.
Based on the CAD model of the test abutment, 70 standardized dies were fabricated with a resin-based 3D printable material (VeroDent MED670; Stratasys, Eden Prairie, MN, USA), using PolyJet technology (Objet Eden500V; Stratasys) in a high-quality build mode with a resolution of 0.0006 inch thick. The build resolution of the 3D printer was as follows: X-axis: 600 dpi; Y-axis: 600 dpi; Z-axis: 1600 dpi. The resin-based printable material used for the die fabrication showed approximately 2000 – 3000 MPa in modulus of elasticity based on the manufacturer's information.
Prior to the cementation, the inner surface of each ceramic crown was treated according to the manufacturers' recommendations. For SU and CD groups, the inner surfaces of the crowns were washed with ethanol, treated with 5% hydrofluoric acid gel (Ceramic etching gel; Ivoclar Vivadent) for 20 seconds, and then washed with water. After removing the residual acid with water, they were air-dried and coated with silane (Monobond N; Ivoclar Vivadent). For EN group, the inner surfaces were washed with ethanol, treated with 5% hydrofluoric acid gel (Ceramic etching gel; Ivoclar Vivadent) for 60 seconds, and then washed with water for 60 seconds. Afterwards, they were air-dried for 20 seconds and coated with silane (Monobond N; Ivoclar Vivadent). For EM group, the inner surfaces were treated with 5% hydrofluoric acid gel (Ceramic etching gel; Ivoclar Vivadent) for 20 seconds, washed with water and air-dried, and coated with silane (Monobond N; Ivoclar Vivadent). A bonding surface of test supporting die was etched with 40% phosphoric acid for 20 seconds, rinsed with water, andair-dried. An adhesive (Scotchbond Universal Adhesive; 3M ESPE, MN, USA) was applied on the bonding surface of each supporting die and cured. Subsequently, a self-adhesive resin cement (RelyX U200; 3M ESPE) was used to bond the crowns to the supporting dies according to the manufacturer's protocol. Before the mechanical testing, the bonded die/crown specimens were stored in distilled water at 37℃ for 24 hours.
A cylinder-shaped test jig was designed using a commercial CAD software (PowerSHAPE; Delcam) to mount the bonded supporting die/crown specimens for the mechanical testing. Based on the CAD data, a customized jig was fabricated with a PLA (polylactic acid) filament (Plaghetti 3D filament, make3D, Seoul, Korea) and a custom 3D printer using fused deposition manufacturing technology. Based on the manufacturer's information, the flexural strength and modulus of elasticity of the FDM filament were 70 MPa and 3212 MPa, respectively. The supporting die/crown specimen was then fixed using a test jig to the universal testing machine (Instron 4467; Instron, Norwood, MA, USA) with a load cell (force sensor) of 10,000 N to measure the fracture load values. The 10-mm-diameter stainless-steel spherical indenter was in contact with the central fossa of the crown's occlusal surface, and a 2-mm-thick urethane rubber sheet was interposed between the indenter and the specimen. Compressive loads were applied to the occlusal surface in a vertical direction with a crosshead speed of 1.0 mm/minute until fracture, at which time the load value (N) at the failure was recorded as fracture resistance to compare with the physiologic masticatory forces in the clinical situation.
After the testing, the fractured surface of each specimen was observed under a stereomicroscope at a magnification of ×40, and two representative specimens from each group were selected for the fractographic analysis. The fractographic analysis was conducted to examine the fracture characteristics of the various ceramic crowns and to identify the crack propagation direction, under a scanning electron microscope (S2300; Hitachi High-Technologies Corporation, Tokyo, Japan) with magnification of ×18.
The means and standard deviations of the fracture load values of all specimens were calculated. For the data of all the groups, Levene's test was used to verify the homogeneity of variances. A two-way analysis of variance (ANOVA) was performed to determine the effects of two factors, the type of ceramics and the occlusal thickness on the fracture resistance of monolithic crowns, and their interactions. In case of significant statistical interaction, a test of simple effects was conducted using a pairwise comparison corrected with the Bonferroni's method. In addition, to identify the differences in the fracture loads among the different crowns with the recommended thickness (1.5 mm), including the control group, a one-way ANOVA was performed. For post-hoc analysis, Tukey's honest significant difference (HSD) test was used. To identify the differences in the fracture resistance of crowns of identical ceramics in relation to occlusal thickness, the independent t-tests were also conducted. All of the statistical analyses were performed using R software (R Foundation for Statistical Computing; Vienna, Austria). The level of statistical significance was set at 0.05.
The means and standard deviations of measured fracture loads of the seven experimental groups and the control group are as follows: 1) for the ceramic groups with reduced occlusal thickness conditions (1.0 mm): 1,494.9 ± 125.9 N (EN), 1,446.5 ± 210.2 N (SU), and 1,467.1 ± 166.1 N (CD); 2) for the ceramic groups with recommended occlusal thickness conditions (1.5 mm): 1,567.0 ± 235.1 N (EN), 2,203.5 ± 371.1 N (SU), 1,795.8 ± 267.6 N (CD); 3) for the control group (EM) with an occlusal thickness of 1.5 mm: 2,437.6 ± 144.4 N (Fig. 2 and Fig. 3).
Levene's test showed that the data in all of the groups satisfied the requirement of homogeneity of variances (P > .05). A two-way ANOVA on the type of ceramics and the amount of occlusal thickness showed a significant interaction between the two factors (P < .001). Based on the pairwise comparison for the test of simple effects, the fracture resistances of monolithic ceramic crowns with reduced occlusal thicknesses (1.0 mm) did not differ significantly, whereas those with recommended thicknesses (1.5 mm) differed significantly (Table 2). For the groups with the same thickness (1.5 mm, Fig. 2), the post-hoc Tukey HSD test showed that the fracture load values were significantly lower in the EN and CD groups than in the SU and EM (control) groups, respectively (P < .05). The mean value of the SU group was not significantly different from that of the control (P = .222). The mean values of the EN and CD groups did not differ significantly (P = .240). For the thickness changes in the identical monolithic ceramic crowns (Fig. 3), the mean fracture load of the SU and CD groups increased significantly when the occlusal thickness increased (P < .001 and P = .004, respectively), whereas that of the EN group did not (P = .403).
The SEM analysis of the fractured surfaces revealed that all the test groups showed multiple crack propagations, arrest lines, compression curls, and hackle lines (twist hackle marks) (Figs. 4, 5, 6, 7, 8). The primary fracture origins were located at the occlusal surfaces of the EN and EM crowns (Fig. 4, Fig. 5, and Fig. 8), while the fracture origins of SU and CD groups were located at the deep inner regions of crowns near the cementation layer (Fig. 6 and Fig. 7). For the EN crowns, the cracks started from the contact point of the loading surface and then stopped in deep occlusal surfaces. For the EM crown, multiple cracks were formed from the occlusal loading point. For the SU and CD crowns, main fracture origins were found at the interfaces between crown and cementation layer and the cracks propagated to the outer proximal or cervical area of the specimens. Even though some small porosities were observed, the overall bonded interface between the test supporting die and the crown showed intimate contact (Figs. 4, 5, 6, 7, 8).
The fracture loads of monolithic crowns were differently influenced by the changes in occlusal thickness, depending on the type of ceramics. When the occlusal thickness was identical, glass ceramic-based crowns showed higher fracture resistance than did the PICN crowns, whereas lithium disilicate crowns had the highest value. Among the ZLS group, only the fracture resistance of the SU crown was similar to the EM crown. Therefore, the null hypothesis of the present study was rejected. In this study, the mean fracture load values of all tested ceramic groups ranged from 1400 N to 2500 N. This suggested that all the monolithic crowns used in the present research were sufficient for clinical use because they can experimentally withstand the average (700 N) or the maximum physiological masticatory forces (1000 N) exerted on posterior human teeth.252627 This holds true even when their occlusal thicknesses have to be lesser (1.0 mm) than those recommended by the manufacturer. These findings, however, may not be directly applied to the clinical situations since additional considerations should be given to the patient's masticatory pattern, antagonistic arch, and the characteristic of the abutment.
The PICN (EN group) has higher fracture resistances and damage tolerances than do pure-phase substances, mostly because of the strengthening mechanism of crack bridge zone formation, plastic deformation, and secondary cracking.28 Since the occlusal thickness affects the fracture resistance of the ceramic crowns, the recommended thickness ranges from 1.3 mm to 2.0 mm.2930 In this study, we tested two different situation of occlusal reduction; 1.5 mm, a recommended thickness, and 1.0 mm, a reduced thickness. Interestingly, the present study showed that an occlusal thickness change in the EN group did not significantly influence its fracture resistance. A composite of polymer and ceramic material may have different physical characteristics with the ceramic component only.30 A simplified molar onlay study using a resin nanoceramic did not show a significant difference in fracture resistance even when the thickness changed from 1.0 mm to 1.5 mm.30 There was no linear relation between fracture load and thickness of the material.30 This differed from the results obtained for lithium disilicate, which showed a linear relation between thickness and fracture load.30 The researchers suggested that the different mechanical properties of the resin matrix and ceramic particles caused the fracture resistance to remain consistent with thickness changes within a certain range.30 Likewise, the stress distribution and fracture mechanism of PICN may be different from those of glass ceramics due to elastic polymer network in this study, which requires further evaluation.
In a previous study of Preis and his colleagues, the ZLS and lithium disilicate crowns on the extracted molars showed similar fracture resistance.31 This was partly in agreement with the findings of the present study. Of the two types of ZLS, only the SU showed fracture resistance comparable to that of lithium disilicate, whereas the CD group showed a significantly lower value compared to the control. Changes in the restoration thickness significantly affected the mean fracture loads of both SU and CD groups. The SU crowns showed higher fracture resistances than the CD crowns with a statistical significance. Based on the instructions of the manufacturers, the SU requires milling in the precrystallized stage and subsequent crystallization firing, whereas the CD starts milling with fully crystallized ceramic block. The manufacturer's data report that most of the physical properties of SU and CD groups, except for the coefficient of thermal expansion, are basically the same. Because ceramics are susceptible to surface flaws and cracks introduced during machining, different fabrication methods between two ZLSs might affect the physical properties or the fracture mechanism of the milled crowns.29 Further controlled laboratory researches on this issue should be followed.
The supporting dies used in the present study were fully generated with CAD/CAM to have the same amount of occlusal and axial reduction. The researchers wanted to ensure that the physical properties of the supporting toothlike structures used in the fracture testing were the same, as previously documented.32 Previously reported load-to-failure test protocols of single ceramic restorations included abutments of natural teeth, composite resin layering, or CAD/CAM.33343536 Each approach has its own advantages and weaknesses with respect to the standardization of the specimens and the physical properties of the supporting dies.3738 To reflect clinical conditions, the physical properties of the supporting die should be similar to those of natural tooth; in particular, the elastic modulus of supporting structure exerts a significant influence on the fracture resistance of ceramic crowns.37 A previous study revealed that the fracture resistance of all-ceramic crown increased with the elastic modulus of the supporting die.39 To simulate the in vivo situation, a supporting die with a low elastic modulus may be suitable for fracture strength test.37 According to the manufacurer's information, the material used for supporting die had an elastic modulus of approximately 3000 MPa. Based on the previous studies using controlled compression tests and nanoindentation tests, the modulus of elasticity of this supporting die was in the range of the reported values of the elastic modulus of healthy human tooth dentin.404142 In addition, the following methods of determining the fracture loads and patterns of monolithic crowns were applied in this study to simulate clinically relevant events: 1) the ceramic crown was cemented on top of a resin-based die; 2) the sample was soaked in distilled water at 37℃ for 24 hours; 3) a rubber film was placed; and 4) the fracture load was tested with a stainless-steel ball.43
To date, many studies have reported fracture loads of lithium disilicate, PICN, and ZLS crowns measured under varying restoration thicknesses and die structures.3144454647 Although it is difficult to compare the measurements between the studies due to variations in the supporting die material, load angle, fatigue load, specimen thickness, and cementation techniques, the measured load values in the present study were within the range of previously reported ones.3144454647 With respect to axial wall thickness, Seydler et al.48 reported that the fracture loads of lithium disilicate crowns were not affected significantly when the axial wall thickness was changed from 1.0 mm to 1.5 mm. However, because the mechanical properties of all the tested ceramics differ, additional studies should focus on the effect of axial thickness assuming minimal tooth reductions on the fracture resistance of various CAD/CAM-generated ceramic crowns.
In the present study, the PICN and lithium disilicate crowns showed that the fractures originated in the area of loading contact, extended toward the cervical area, and resulted in a pattern of complete separation while the crown was still attached to the die. In the EN sample, the propagation of minor crack was stopped within the deep occlusal surface, suggesting possible crack limitation due to good damage tolerance. In accordance with our findings, recent studies showed most of the fractures involved both the abutments and the crowns when monolithic ceramics or composites were cemented to supporting resin dies or bovine teeth.4950 The crack propagations in those studies were dominantly from occlusal to cervical direction.434950 On the contrary, the ZLS crowns tested in our study showed different patterns of fractures, with deeply located crack origins near the interfaces between the crown and cementation layer. The compression curls and twisted hackle marks with bifurcations suggested possible crack propagation across the occlusal surface. Since all the tested crowns were destructed catastrophically, the fracture origins of ZLS group detected in this fractographic analysis could be regarded as the primary. Moreover, the measured fracture load values of SU crowns were similar to the previously reported ones in other ZLS-crown study.31 Clinical trials with complex designs should be required to validate the mechanical behaviors of ceramic crowns in oral environments. However, laboratory studies with controlled factors are still able to provide clinical relevant data if proper conduction and interpretation were guaranteed.4950 Considering the difficulties of standardizing the properties and forms of natural teeth specimens, we performed our experiments as a preclinical pilot study with standardized abutments. There are limitations in applying the current findings to clinical settings, further assessments in controlled clinical studies are needed.
The present study measured the fracture resistance of the monolithic ceramic blocks without considering the fatigue of ceramics and loading direction (lateral force). The mechanical test was simply conducted after storing the specimens in distilled water for 24 hours, without thermal or mechanical cyclic loading. Therefore, the results of this study can only provide limited information of the initial performances of the ceramic crowns. Since proper reduction of the tooth structure is essential for the optimal strength, shade, and retention of the finished ceramic restorations, future studies should impose more stringent thickness limits and load-bearing capacities, considering various parameters. As mentioned earlier, experimental studies exclude many of the variables that are found in clinical settings; thus, long-term assessments of these materials in clinical trials should be conducted according to patient characteristics, locations, masticatory patterns, antagonistic conditions, and large sample size.
Within the limitations of the study, the fracture loads of monolithic crowns were differently influenced by the changes in occlusal thickness, depending on the type of ceramics. Comparisons between different ceramic blocks showed that the fracture resistances of the SU and EM crowns were comparable. The fracture load was significantly lower for the PICN group than for the lithium disilicate group. However, all the tested monolithic crowns had higher values than the maximum physiological masticatory force both at the recommended and reduced occlusal thickness, thereby demonstrating their clinical applicability in posterior esthetic restorations.
Figures and Tables
 | Fig. 1Computer-aided-design (CAD) models of test supporting die (A) and definitive molar crowns with occlusal thicknesses of 1.0 mm (B). |
 | Fig. 2Mean and standard deviations of fracture loads of ceramic groups with the same occlusal thickness (1.5 mm). Significant differences (P < .05) between the groups were evaluated with Tukey HSD test and marked with asterisks (*). EN - Enamic, polymer-infiltrated-ceramic network, SU - Suprinity, zirconia-reinforced lithium silicate, CD - Celtra Duo, zirconia-reinforced lithium silicate, EM - e.max CAD, lithium disilicate. |
 | Fig. 3Means and standard deviations of fracture loads of ceramic groups with different occlusal thickness conditions (1.0 mm and 1.5 mm). Significant differences (P < .05) between the groups were evaluated with independent t-tests and marked with asterisks. EN - Enamic, polymer-infiltrated-ceramic network, SU - Suprinity, zirconia-reinforced lithium silicate, CD - Celtra Duo, zirconia-reinforced lithium silicate. |
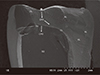 | Fig. 4SEM fracture surface image of EN crown with occlusal thickness of 1.0 mm. HL: hackle line, AL: arrest line, white star: origin of fracture, TH: twist hackle, white solid arrow: crack propagation direction (CPD), CL: cementation layer, SD: supporting die. |
 | Fig. 5SEM fracture surface image of EN crown with occlusal thickness of 1.5 mm. HL: hackle line, AL: arrest line, white star: origin of fracture, white solid arrow: crack propagation direction (CPD), CL: cementation layer, SD: supporting die. |
 | Fig. 6SEM fracture surface image of SU crown with occlusal thickness of 1.5 mm. HL: hackle line, AL: arrest line, CC: compression curl, TH: twist hackle, white star: origin of fracture, white solid arrow: crack propagation direction (CPD), CL: cementation layer, SD: supporting die. |
 | Fig. 7SEM fracture surface image of CD crown with occlusal thickness of 1.5 mm. HL: hackle line, AL: arrest line, CC: compression curl, TH: twist hackle, white star: origin of fracture, white solid arrow: crack propagation direction (CPD), CL: cementation layer, SD: supporting die. |
 | Fig. 8SEM fracture surface image of EM crown with occlusal thickness of 1.5 mm. HL: hackle line, AL: arrest line, CC: compression curl, TH: twist hackle, white star: origin of fracture, white solid arrow: crack propagation direction (CPD), CL: cementation layer, SD: supporting die. |
Table 1
Manufacturers' information of various CAD/CAM ceramic blocks tested in this study

Table 2
Pairwise comparisons of tested ceramic groups with different occlusal thicknesses

References
1. Wang X, Fan D, Swain MV, Zhao K. A systematic review of all-ceramic crowns: clinical fracture rates in relation to restored tooth type. Int J Prosthodont. 2012; 25:441–450.
2. Pjetursson BE, Sailer I, Zwahlen M, Hämmerle CH. A systematic review of the survival and complication rates of allceramic and metal-ceramic reconstructions after an observation period of at least 3 years. Part I: Single crowns. Clin Oral Implants Res. 2007; 18:73–85.
3. Raigrodski AJ, Hillstead MB, Meng GK, Chung KH. Survival and complications of zirconia-based fixed dental prostheses: a systematic review. J Prosthet Dent. 2012; 107:170–177.
4. Sailer I, Makarov NA, Thoma DS, Zwahlen M, Pjetursson BE. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part I: Single crowns (SCs). Dent Mater. 2015; 31:603–623.
5. Miyazaki T, Nakamura T, Matsumura H, Ban S, Kobayashi T. Current status of zirconia restoration. J Prosthodont Res. 2013; 57:236–261.
6. Raigrodski AJ, Yu A, Chiche GJ, Hochstedler JL, Mancl LA, Mohamed SE. Clinical efficacy of veneered zirconium dioxide-based posterior partial fixed dental prostheses: five-year results. J Prosthet Dent. 2012; 108:214–222.
7. Pieger S, Salman A, Bidra AS. Clinical outcomes of lithium disilicate single crowns and partial fixed dental prostheses: a systematic review. J Prosthet Dent. 2014; 112:22–30.
8. Agustín-Panadero R, Román-Rodríguez JL, Ferreiroa A, Solá-Ruíz MF, Fons-Font A. Zirconia in fixed prosthesis. A literature review. J Clin Exp Dent. 2014; 6:e66–e73.
9. Larsson C, Wennerberg A. The clinical success of zirconiabased crowns: a systematic review. Int J Prosthodont. 2014; 27:33–43.
10. Beuer F, Stimmelmayr M, Gueth JF, Edelhoff D, Naumann M. In vitro performance of full-contour zirconia single crowns. Dent Mater. 2012; 28:449–456.
11. Denry I, Kelly JR. Emerging ceramic-based materials for dentistry. J Dent Res. 2014; 93:1235–1242.
12. Zhao K, Wei YR, Pan Y, Zhang XP, Swain MV, Guess PC. Influence of veneer and cyclic loading on failure behavior of lithium disilicate glass-ceramic molar crowns. Dent Mater. 2014; 30:164–171.
13. Guess PC, Zavanelli RA, Silva NR, Bonfante EA, Coelho PG, Thompson VP. Monolithic CAD/CAM lithium disilicate versus veneered Y-TZP crowns: comparison of failure modes and reliability after fatigue. Int J Prosthodont. 2010; 23:434–442.
14. Batson ER, Cooper LF, Duqum I, Mendonça G. Clinical outcomes of three different crown systems with CAD/CAM technology. J Prosthet Dent. 2014; 112:770–777.
15. Li RW, Chow TW, Matinlinna JP. Ceramic dental biomaterials and CAD/CAM technology: state of the art. J Prosthodont Res. 2014; 58:208–216.
16. He LH, Swain M. A novel polymer infiltrated ceramic dental material. Dent Mater. 2011; 27:527–534.
17. Coldea A, Swain MV, Thiel N. Mechanical properties of polymer-infiltrated-ceramic-network materials. Dent Mater. 2013; 29:419–426.
18. Coldea A, Swain MV, Thiel N. In-vitro strength degradation of dental ceramics and novel PICN material by sharp indentation. J Mech Behav Biomed Mater. 2013; 26:34–42.
19. Coldea A, Swain MV, Thiel N. Hertzian contact response and damage tolerance of dental ceramics. J Mech Behav Biomed Mater. 2014; 34:124–133.
20. Mörmann WH, Stawarczyk B, Ender A, Sener B, Attin T, Mehl A. Wear characteristics of current aesthetic dental restorative CAD/CAM materials: two-body wear, gloss retention, roughness and Martens hardness. J Mech Behav Biomed Mater. 2013; 20:113–125.
21. Coldea A, Fischer J, Swain MV, Thiel N. Damage tolerance of indirect restorative materials (including PICN) after simulated bur adjustments. Dent Mater. 2015; 31:684–694.
22. Ankyu S, Nakamura K, Harada A, Hong G, Kanno T, Niwano Y, Örtengren U, Egusa H. Fatigue analysis of computer-aided design/computer-aided manufacturing resinbased composite vs. lithium disilicate glass-ceramic. Eur J Oral Sci. 2016; 124:387–395.
23. Zesewitz TF, Knauber AW, Northdurft FP. Fracture resistance of a selection of full-contour all-ceramic crowns: an in vitro study. Int J Prosthodont. 2014; 27:264–266.
24. Güncü MB, Cakan U, Muhtarogullari M, Canay S. Zirconiabased crowns up to 5 years in function: a retrospective clinical study and evaluation of prosthetic restorations and failures. Int J Prosthodont. 2015; 28:152–157.
25. Kikuchi M, Korioth TW, Hannam AG. The association among occlusal contacts, clenching effort, and bite force distribution in man. J Dent Res. 1997; 76:1316–1325.
26. Ferrario VF, Sforza C, Zanotti G, Tartaglia GM. Maximal bite forces in healthy young adults as predicted by surface electromyography. J Dent. 2004; 32:451–457.
27. Varga S, Spalj S, Lapter Varga M, Anic Milosevic S, Mestrovic S, Slaj M. Maximum voluntary molar bite force in subjects with normal occlusion. Eur J Orthod. 2011; 33:427–433.
28. Wegner LD, Gibson LJ. The fracture toughness behaviour of interpenetrating phase composites. Int J Mech Sci. 2001; 43:1771–1791.
29. Thompson VP, Rekow DE. Dental ceramics and the molar crown testing ground. J Appl Oral Sci. 2004; 12:26–36.
30. Chen C, Trindade FZ, de Jager N, Kleverlaan CJ, Feilzer AJ. The fracture resistance of a CAD/CAM Resin Nano Ceramic (RNC) and a CAD ceramic at different thicknesses. Dent Mater. 2014; 30:954–962.
31. Preis V, Behr M, Hahnel S, Rosentritt M. Influence of cementation on in vitro performance, marginal adaptation and fracture resistance of CAD/CAM-fabricated ZLS molar crowns. Dent Mater. 2015; 31:1363–1369.
32. Nordahl N, Vult von, Larsson C. Fracture strength of ceramic monolithic crown systems of different thickness. J Oral Sci. 2015; 57:255–261.
33. Schultheis S, Strub JR, Gerds TA, Guess PC. Monolithic and bi-layer CAD/CAM lithium-disilicate versus metal-ceramic fixed dental prostheses: comparison of fracture loads and failure modes after fatigue. Clin Oral Investig. 2013; 17:1407–1413.
34. Coelho PG, Bonfante EA, Silva NR, Rekow ED, Thompson VP. Laboratory simulation of Y-TZP all-ceramic crown clinical failures. J Dent Res. 2009; 88:382–386.
35. Choi YS, Kim SH, Lee JB, Han JS, Yeo IS. In vitro evaluation of fracture strength of zirconia restoration veneered with various ceramic materials. J Adv Prosthodont. 2012; 4:162–169.
36. Harada A, Nakamura K, Kanno T, Inagaki R, Örtengren U, Niwano Y, Sasaki K, Egusa H. Fracture resistance of computer-aided design/computer-aided manufacturing-generated composite resin-based molar crowns. Eur J Oral Sci. 2015; 123:122–129.
37. Yucel MT, Yondem I, Aykent F, Eraslan O. Influence of the supporting die structures on the fracture strength of all-ceramic materials. Clin Oral Investig. 2012; 16:1105–1110.
38. Dittmer MP, Kohorst P, Borchers L, Stiesch M. Influence of the supporting structure on stress distribution in all-ceramic FPDs. Int J Prosthodont. 2010; 23:63–68.
39. Scherrer SS, de Rijk WG. The fracture resistance of all-ceramic crowns on supporting structures with different elastic moduli. Int J Prosthodont. 1993; 6:462–467.
40. Chun K, Choi H, Lee J. Comparison of mechanical property and role between enamel and dentin in the human teeth. J Dent Biomech. 2014; 5:1758736014520809.
41. Stanford WJ, Paffenbarger GC, Kumpula JW, Sweeney WT. Determination of some compressive properties of human enamel and dentin. J Am Dent Assoc. 1958; 57:487–495.
42. Arcís RW, López-Macipe A, Toledano M, Osorio E, Rodríguez-Clemente R, Murtra J, Fanovich MA, Pascual CD. Mechanical properties of visible light-cured resins reinforced with hydroxyapatite for dental restoration. Dent Mater. 2002; 18:49–57.
43. Øilo M, Kvam K, Tibballs JE, Gjerdet NR. Clinically relevant fracture testing of all-ceramic crowns. Dent Mater. 2013; 29:815–823.
44. Nakamura K, Harada A, Inagaki R, Kanno T, Niwano Y, Milleding P, Ortengren U. Fracture resistance of monolithic zirconia molar crowns with reduced thickness. Acta Odontol Scand. 2015; 73:602–608.
45. Johansson C, Kmet G, Rivera J, Larsson C, Vult Von. Fracture strength of monolithic all-ceramic crowns made of high translucent yttrium oxide-stabilized zirconium dioxide compared to porcelain-veneered crowns and lithium disilicate crowns. Acta Odontol Scand. 2014; 72:145–153.
46. Sun T, Zhou S, Lai R, Liu R, Ma S, Zhou Z, Longquan S. Load-bearing capacity and the recommended thickness of dental monolithic zirconia single crowns. J Mech Behav Biomed Mater. 2014; 35:93–101.
47. de Kok P, Kleverlaan CJ, de Jager N, Kuijs R, Feilzer AJ. Mechanical performance of implant-supported posterior crowns. J Prosthet Dent. 2015; 114:59–66.
48. Seydler B, Rues S, Müller D, Schmitter M. In vitro fracture load of monolithic lithium disilicate ceramic molar crowns with different wall thicknesses. Clin Oral Investig. 2014; 18:1165–1171.
49. Campos RE, Soares PV, Versluis A, de O, Ambrosano GM, Nunes IF. Crown fracture: Failure load, stress distribution, and fractographic analysis. J Prosthet Dent. 2015; 114:447–455.
50. Harada A, Nakamura K, Kanno T, Inagaki R, Örtengren U, Niwano Y, Sasaki K, Egusa H. Fracture resistance of computer-aided design/computer-aided manufacturing-generated composite resin-based molar crowns. Eur J Oral Sci. 2015; 123:122–129.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download