Abstract
PURPOSE
The purpose of this study was to determine fracture resistance and failure modes of three-unit fixed dental prostheses (FDPs) made of lithium disilicate pressed on zirconia (LZ), monolithic lithium disilicate (ML), and monolithic zirconia (MZ).
MATERIALS AND METHODS
Co-Cr alloy three-unit metal FDPs model with maxillary first premolar and first molar abutments was fabricated. Three different FDPs groups, LZ, ML, and MZ, were prepared (n = 5 per group). The three-unit FDPs designs were identical for all specimens and cemented with resin cement on the prepared metal model. The region of pontic in FDPs was given 50,000 times of cyclic preloading at 2 Hz via dental chewing simulator and received a static load until fracture with universal testing machine fixed at 10°. The fracture resistance and mode of failure were recorded. Statistical analyses were performed using the Kruskal-Wallis test and Mann-Whitney U test with Bonferroni's correction (α=0.05/3=0.017).
RESULTS
A significant difference in fracture resistance was found between LZ (4943.87 ± 1243.70 N) and ML (2872.61 ± 658.78 N) groups, as well as between ML and MZ (4948.02 ± 974.51 N) groups (P<.05), but no significant difference was found between LZ and MZ groups (P>.05). With regard to fracture pattern, there were three cases of veneer chipping and two interfacial fractures in LZ group, and complete fracture was observed in all the specimens of ML and MZ groups.
Porcelain-fused-to-metal (PFM) restorations have been widely used for fixed dental prostheses (FDPs).1 However, PFM restorations have problems, such as metal exposure at the cervical margin, discoloration of porcelain, potential disadvantage of the alloy, and possible bonding problem between the framework and veneering material.2 Also, they may cause allergy and staining along with the release of metal ions at the gingival tissue.3 These problems of PFM were resolved through the development of all-ceramic restorations without a metal. The usage of all-ceramic crowns in restorative dentistry has been increased due to its natural color, as well as its translucency as in feldspathic porcelain, high biocompatibility, relatively affordable price compared to precious metals, and increasing demand for metal-free FDPs.45
As high-strength ceramic materials and systems have been developed due to the introduction of the computer-aided design/computer-aided manufacturing (CAD/CAM) technologies, the scope of the anterior and posterior FDPs usage has been expanded.6 Among the currently available all-ceramic materials, yttria-stabilized tetragonal zirconia polycrystalline (Y-TZP) shows the greatest fracture resistance, and it is considered as the gold standard in dental crown restorations.7 However, Y-TZP has limitations, including difficult control of its opacity and translucency, chipping of the veneered porcelain, and limited optical defects.8 As so, a lot of efforts, including coloring, have been made to overcome these problems, but the problems still remain. After all, porcelain-fused-to-zirconia (PFZ) has been chosen as suitable zirconia prosthesis.9
The method of veneering the zirconia core using the hand-layering technique with feldspathic porcelain has mainly been used, but as this method is technique sensitive and as veneer chipping often occurs,10 reducing the fracture rate of porcelain veneers has become a major concern in zirconia restorations.11 Due to the better mechanical properties of lithium disilicate ceramic, a transition from feldspathic porcelain to lithium disilicate ceramic for veneer fabrication has been suggested in order to improve the fracture resistance.12 CAD-on technique was used to mill frame and veneer from ceramic blanks. Either by using resin cement or by fusing ceramic, lithium disilicate veneer can be attached to zirconia framework after sintering the framework. 13 Recently, heat-pressed method that presses lithium disilicate glass ceramic veneer has been introduced.14 As the lost wax press system can produce ceramic prostheses with or without CAD/CAM system, it is economical and simple, and it can effectively reconstruct the customized anatomical shape.15
A study utilized lithium disilicate glass ceramic as a heat-pressed veneered ceramic in fabrication of zirconia crown, and it showed a higher fracture resistance than fluroaptite-pressed zirconia crown and monolithic lithium disilicate crown.14 However, there are almost no studies on lithium disilicate pressed zirconia FDPs. Hence, the aim of the present study was to investigate relative fracture resistance and failure mode of three-unit FDPs made of lithium disilicate pressed on zirconia, monolithic lithium disilicate, and monolithic zirconia.
A master model with two abutments in the maxillary first premolar and first molar was fabricated. To fabricate the master model, each abutment was first designed using CAD software (Exocad, Exocad GmbH, Darmstadt, Germany). Each abutment was fabricated with chamfer preparation of 120° and convergence angle of 15° (Fig. 1A).16 The STL file of the designed master model was transmitted to the five-axis milling machine (Ceramill Motion 2, AmannGirrbach, Koblach, Austria), and dry milling was performed with Co-Cr blanks (Ceramill Sintron, Amann Girrbach, Koblach, Austria). After that, a total of 15 master models were fabricated through sintering at a high-temperature sintering furnace (Ceramill Argotherm, AmannGirrbach, Koblach, Austria) (Fig. 2).
The experiment was performed by dividing the specimen into three groups: the lithium disilicate glass ceramics pressed on zirconia-based FDPs (LZ group), the monolithic lithium disilicate FDPs (ML group), and the monolithic zirconia FDPs (MZ group). All the ceramic materials are presented in Table 1. Three-unit FDPs from the first premolar to the first molar were fabricated, with the same shape as the one used in clinical practice (Fig. 1B). A total of 15 three-unit FDPs, five for each group, were fabricated (Fig. 3). The master models were scanned using the AutoScan 3D Dental Scanner (Hangzhou Shining 3D Tech Co., Ltd., Hangzhou, China), and same-shaped crowns were designed using the Exocad software (Exocad GmbH, Darmstadt, Germany). The connectors included mesiodistal cross-sectional areas of 31.3 mm2, a buccolingual width of 5.7 mm, and an occlusogingival height of 7 mm.17
To fabricate lithium disilicate glass ceramics pressed on zirconia-based FDPs, a 0.5 mm-thick zirconia coping (Zirtooth Fulluster, HASS, Gangneung, Korea) was prepared first. After making the coping, liner powder (Rosetta Ceram Liner, HASS, Gangneung, Korea) was applied to the surface of the zirconia to improve the bond strength and wettability between the zirconia and the veneer glass ceramic, and heat treatment was done according to the instruction of the manufacturer. Using the Exocad software, a wax veneer structure was fabricated by milling a wax block (TOTEM, Qingdao Totem Candle Industry, Shandong, China). The veneer was fixed to the coping by applying heat to its margin, and after investing using a dedicated investment ring, it was burned out at 880℃ for 30 minutes (Burnout Furnace L 1/12, Nabertherm, Bremen, Germany). After that, glass ingot (Rosetta UltraPress, HASS, Gangneung, Korea) was put in the investment ring, and the latter was pressed with the pressing furnace (Horizon Press, Shenpaz Dental Ltd., Migdal HaEmek, Israel) to join the zirconia and the lithium disilicate glass ceramic. According to the instruction of the manufacturer, sandblasting (50 µm glass beads at 1 bar pressure) and glazing (IPS e.max Ceram glaze paste, Ivoclar Vivadent, Schaan, Leichtenstein) were performed. The monolithic lithium disilicate FDPs were fabricated by obtaining same-shaped wax patterns by milling the wax block, pressing the lithium disilicate ingots (Rosetta SuperPress, HASS, Gangneung, Korea) using the heat-pressing technique, and glazing (IPS e.max Ceram glaze paste, Ivoclar Vivadent, Schaan, Leichtenstein). Then, the monolithic zirconia FDPs were fabricated through milling with a milling machine (Roland DWX-50, Roland DGA, Irvine, CA, USA), final-sintering, and glazing (IPS e.max Ceram glaze paste, Ivoclar Vivadent, Schaan, Leichtenstein). For the purpose of standardization, the same dental technician executed the manufacturing process.
Cementation of the FDPs was done in the master model using G-CEM LinkAce resin cement (GC America, Alsip, IL, USA), and the samples were kept in distilled water at 37℃ for more than 48 hours. To preload the FDPs, 50,000 times of mechanical loading were applied on the occlusal surface of the pontic under 50 N at 2 Hz, using dental chewing simulator (R&D Inc., Daejeon, Korea) (Fig. 4A). The load was applied on the distal part of occlusal surface of the pontic by using a stainless steel sphere with a 4 mm diameter. Lastly, the FDPs were fixed onto the testing jig at 10° angle against the long axis, and load to fracture was applied using a universal testing machine (Instron 8871, Instron Co., Norwood, MA, USA) (Fig. 4B). The crosshead speed was set to 0.255 mm/min, and loading was executed to the previously preloaded site, using a stainless steel sphere with a 4 mm diameter, until the FDPs were fractured.16 The fracture resistance was recorded when the FDPs were fractured; in this present study, fracture was defined as an abrupt force decrease, and the maximum force prior to the sudden decline was recorded as fracture resistance. The fracture pattern was classified into complete fracture, veneer chipping, and interfacial fracture, and the numbers of fracture were counted for each group. The specimens of LZ group were coated with platinum, and their microstructure of fractured surface was observed, using FE-SEM (JSM-7401F, Jeol, Tokyo, Japan) (×180, ×250, and ×700).
To evaluate the statistical significance of the fracture resistance of the FDPs by material, SPSS ver. 23.0 (SPSS Inc., Chicago, IL, USA) was used. For the comparison of the three groups, the Kruskal-Wallis and Mann-Whitney U tests with Bonferroni's correction (α = .05/3 = .017) were employed. All statistical analyses were done at the 5% significance level.
Preloading process did not induce fracture on all FDPs. The statistical analysis showed a significant difference between the LZ group (4943.87 ± 1243.70 N) and the ML group (2872.61 ± 658.78 N) as well as between the ML and MZ groups (4948.02 ± 974.51 N) (P < .05), but it did not exhibit a significant difference between the LZ and MZ groups (P > .05) (Fig. 5). In terms of fracture pattern, three cases of veneer chipping and two interfacial fractures in the LZ group and complete fracture in all the specimens of ML and MZ groups were observed (Fig. 6, Table 2). The fracture lines of the specimens in the LZ group were formed vertically along the load direction, and the fracture pattern was clearly seen at the pontic where a direct force was given. In the ML and MZ groups, most of the fracture patterns were also formed in the mesiodistal direction, centering on the pontic where a direct force was given. The specimen that experienced veneer chipping in the LZ group was observed with FE-SEM, displaying no pore, defect, or void between zirconia core and lithium disilicate glass ceramic veneer (Fig. 7).
Preclinical in vitro tests for dental materials are important in evaluating mechanical performance and compatibility of materials in mouth.18 The conventional laboratory tests apply static loading until failure using a universal testing machine. These tests provide information on material strength, potential risk of failure, and deformation of the material. However, they cannot sufficiently predict the longterm performance of dental restorations.19 The intraoral environments that need to be considered for dental restorations include humidity, pH, and cyclic loading. Therefore, studies must reproduce experimental conditions similar to the clinical situations in order to create the failure pattern in actual clinical practice.1819
In the present study, the FDPs were designed to mimic the clinical situation anatomically as much as possible. Herein, the loading stainless steel ball was able to fit in a cavity formed in the middle of the pontic, and a three-point contact between the occlusal surface and steel ball was achieved successfully.17 Clinically, the mechanical failure of dental prostheses occurs a long time after their application, indicating that fatigue failure accounts for a larger proportion of the failure cases than acute overload.20 Damage is accumulated by repetitive contact between maxillary and mandibular teeth, and the lifetime and survival rate of the prosthesis are reduced.21 Therefore, this study performed a fatigue testing in order to replicate the clinical settings as much as possible.18 The preload force was set at 50 N, based on the previous studies.622 50,000 cycles were applied during the preload to replicate the average number of mastication in 4 weeks.23 Moreover, the preloading was conducted in dry environment. Thermal cycling with water removes water from the surface of specimens and leads to further aging of material. In addition, wet environment may cause subcritical crack growth, change in ceramic structure, and superficial phase transitions.24 Therefore, less fracture resistance might be speculated if the experimental condition was set in wet environment. Furthermore, as most of the previous studies reported a chewing rate in humans under 2 Hz, the test frequency of this study was set at 2 Hz.2526 Nevertheless, because it is impossible for in vitro studies to replicate clinical scenario completely, the result of the experiment may be different from that in the actual clinical setting. For instance, the present experimental model did not employ periodontal ligaments while the periodontal ligaments around roots are responsible for stress distribution in natural dentition.27 As shown in the previous study, the models without periodontal ligament demonstrated a greater fracture resistance than the models with periodontal ligament.22 In addition, natural tooth would have replicated the clinical settings more precisely if chosen as an abutment of this study. Yet, the present study chose metal alloy abutment since natural teeth have different size, shape, and quality, and the preparation cannot be standardized.282930 In addition, natural teeth with lower elastic modulus can be fractured near cervical area.3132 Therefore, the present study utilized metal alloy abutment with higher elastic modulus and fracture resistance for testing fracture resistance of the experimental groups. Resin cement with strong rigidity was also used to lower a chance of failure by luting agent.33 Moreover, the influence of elastic modulus of abutment on the fracture resistance of dental prosthesis was demonstrated in many in vitro studies,293134 and some clinical studies reported a higher failure rate of crown in response to lower elastic modulus of abutment material.35 Therefore, based on the earlier studies, the experimental outcomes of the present study are thought to manifest higher fracture resistance than in the clinical situation. However, the present study rather investigated relative comparison among the fracture resistances of the experimental groups, regardless of the experimental condition. Therefore, these factors may not affect the relative comparison among the groups.
The ML group in this experiment was lithium disilicate glass ceramic, a kind of particle-filled glass that has been reported to have a higher fracture resistance than leucite-reinforced glass ceramic or feldspathic ceramic.36 The reason for this seems to be that lithium disilicate crystal is more efficient in promoting crack deflection and crack branching compared to glass ceramic including the leucite or fluorapatite crystal phase.3738 The MZ group consists of the typical yttria-stabilized tetragonal zirconia polycrystalline (Y-TZP) and provides high toughness and fracture load through the mechanism called “transformation toughening.”39 In other words, when the phase changes from the tetragonal phase to the monoclinic phase, 3 - 5% volume expansion occurs, which gives rise to internal stress.40 Stabilizing agents such as calcium, magnesia, yttrium, and ceria are also added in order to stabilize the tetragonal phase at room temperature and to control the volume expansion.41 In this study, the fracture resistance of the MZ group (4948.02 ± 974.51 N) was significantly larger than that of the ML group (2872.61 ± 658.78 N), which is consistent with the results of other previous studies.11442 This is because polycrystalline materials are less vulnerable to fatigue degradation than glass ceramic.43 In a previous study, it was confirmed that the crack propagation of lithium disilicate glass ceramic appeared only within the residual glass matrix and did not spread through the crystal.38 Additionally, such result can be partially explained by the flexural strengths of lithium disilicate glass ceramic and zirconia (440 and 1250 MPa, respectively). In this study, the fracture resistance of the monolithic zirconia FDPs (4948.02 ± 974.51 N) and the lithium disilicate glass ceramics pressed on zirconia-based FDPs (4943.87 ± 1243.70 N) did not show a statistically significant difference, which is similar to the result of another study that compared crowns.14 Also, a comparable level of fracture resistance in both lithium desilicated glass ceramics pressed on zirconia-based FDPs and monolithic zirconia FDPs accounts that a higher fracture resistance of FDPs with ceramic core can be obtained from the core material.30
Fracture can be roughly divided into delamination (core exposure and adhesive facture), cohesive fracture within the veneer porcelain (chipping), cracks extending to the framework (radial cracks), and complete fracture (catastrophic fracture, bulk fracture, and total fracture).11 In this study, the ML and MZ groups, which were monolithic FDPs, showed complete fracture while the LZ group showed veneer chipping and interfacial fracture. From the clinial perspective, chipping is mainly observed at the site where a high contact force is applied, and it appears more when air bubbles are present inside the veneer layer. In fact, during the fabrication of the conventional feldspathic ceramic veneer, pores are inevitably generated, but the fabrication through press system prevents the formation of these microstructural defects.44 As evident in Fig. 7, the secure contact between lithium disilicate glass ceramic veneers and zirconia core may be responsible for the high fracture resistance. Yet, considering that, in some specimens, interfacial fracture was observed between the core and the veneer and that the standard deviation of the fracture resistance of the LZ group was greater than those of the two other groups, further studies must be performed on the reasons for the higher fracture resistance.
Meanwhile, many studies on the occlusal force reported that gender, age, and measurement site including the anterior and posterior regions resulted in the considerably different values. Dental restoration must be able to support occlusal force greater than 1000 N because the occlusal force exceeds more than 1000 N in parafuction.3039 Within the limitation of this study, as all the groups showed a fracture resistance of more than 1000 N, the fracture resistance can be clinically accepted.
As the limitation of this study, the oral environment displays thermal stress due to the presence of water while the present experiment was performed in dry environment. In addition, mechanical property of the metal alloy abutment was different from that of natural teeth, the load was executed in a single direction, and the absence of periodontal ligament did not represent the clinical settings completely. Moreover, there are limitations because of the small number of specimens, and there is a need for additional experimental groups such as CAD-fabricated lithium disilicate veneer luted or fused on zirconia-framework. Therefore, further studies should be executed to investigate the additional groups of FDPs mentioned above and long-term effects of cyclic loading under thermal stress along with clinical studies.
Within the limitations of this study, such as the small number of the specimens, lithium disilicate glass ceramics pressed on zirconia-based FDPs showed comparable fracture resistance to monolithic zirconia FDPs and more outstanding fracture resistance than monolithic lithium disilicate FDPs.
Figures and Tables
Fig. 2
Preparation of the master model. (A) STL file of the master model, (B) Front view of the fabricated alloy master model, (C) Occlusal view of the fabricated alloy master model.

Fig. 3
Fabrication of three-unit FDPs. (A) LZ group: lithium disilicate glass ceramics pressed on zirconia-based FDPs, (B) ML group: monolithic lithium disilicate FDPs, (C) MZ group: monolithic zirconia FDPs.

Fig. 4
Preparation of the test set-up. (A) Repetitive preloading in a chewing simulator, (B) Fracture load test in a universal testing machine.

Fig. 5
Box plots of fracture resistance for each experimental group. The same lowercase letter suggests no significant difference found among the groups (P > .05). LZ: lithium disilicate glass ceramic pressed on zirconia, ML: monolithic lithium disilicate, MZ: monolithic zirconia.

Fig. 6
Typical failure types of the experimental groups after the fracture load test. (A) Veneer chipping of the LZ group, (B) Interfacial fracture of the LZ group, (C) Complete fracture of the ML group, (D) Complete fracture of the MZ group. LZ: lithium disilicate glass ceramic pressed on zirconia, ML: monolithic lithium disilicate, MZ: monolithic zirconia.
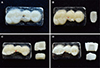
Fig. 7
SEM image of the specimen with veneer chipping in LZ group. (A) No pore, defect, or void observed between zirconia core and lithium dislicate glass ceramic veneer, (B) Magnified image of area inside rectangle in Fig. 7A, (C) Magnified image of area inside rectangle in Fig. 7B (original magnification: ×180, ×250, ×700, respectively). Zr: zirconia coping, Li: lithium disilicate glass ceramic veneer.

Table 1
Ceramic materials used in this study, and properties of each group

References
1. Lüthy H, Filser F, Loeffel O, Schumacher M, Gauckler LJ, Hammerle CH. Strength and reliability of four-unit all-ceramic posterior bridges. Dent Mater. 2005; 21:930–937.
2. Datla SR, Alla RK, Alluri VR, Babu JP, Konakanchi A. Dental ceramics: Part II - Recent advances in dental ceramics. Am J Mater Eng Technol. 2015; 3:19–26.
3. Venclíkova Z, Benada O, Bártova J, Joska L, Mrklas L. Metallic pigmentation of human teeth and gingiva: morphological and immunological aspects. Dent Mater J. 2007; 26:96–104.
4. Donovan TE. Factors essential for successful all-ceramic restorations. J Am Dent Assoc. 2008; 139:14S–18S.
5. Sailer I, Fehér A, Filser F, Gauckler LJ, Lüthy H, Hämmerle CH. Five-year clinical results of zirconia frameworks for posterior fixed partial dentures. Int J Prosthodont. 2007; 20:383–388.
6. Schultheis S, Strub JR, Gerds TA, Guess PC. Monolithic and bi-layer CAD/CAM lithium-disilicate versus metal-ceramic fixed dental prostheses: comparison of fracture loads and failure modes after fatigue. Clin Oral Investig. 2013; 17:1407–1413.
7. Rekow ED, Silva NR, Coelho PG, Zhang Y, Guess P, Thompson VP. Performance of dental ceramics: challenges for improvements. J Dent Res. 2011; 90:937–952.
8. Kim HK, Kim SH. Effect of the number of coloring liquid applications on the optical properties of monolithic zirconia. Dent Mater. 2014; 30:e229–e237.
9. Takeichi T, Katsoulis J, Blatz MB. Clinical outcome of single porcelain-fused-to-zirconium dioxide crowns: a systematic review. J Prosthet Dent. 2013; 110:455–461.
10. Sailer I, Gottnerb J, Kanelb S, Hammerle CH. Randomized controlled clinical trial of zirconia-ceramic and metal-ceramic posterior fixed dental prostheses: a 3-year follow-up. Int J Prosthodont. 2009; 22:553–560.
11. Silva NR, Bonfante EA, Rafferty BT, Zavanelli RA, Rekow ED, Thompson VP, Coelho PG. Modified Y-TZP core design improves all-ceramic crown reliability. J Dent Res. 2011; 90:104–108.
12. Baltzer A. All-ceramic single-tooth restorations: choosing the material to match the preparation-preparing the tooth to match the material. Int J Comput Dent. 2008; 11:241–256.
13. Schmitter M, Schweiger M, Mueller D, Rues S. Effect on in vitro fracture resistance of the technique used to attach lithium disilicate ceramic veneer to zirconia frameworks. Dent Mater. 2014; 30:122–130.
14. Kim SY, Choi JW, Ju SW, Ahn JS, Yoon MJ, Huh JB. Fracture strength after fatigue loading of lithium disilicate pressed zirconia crowns. Int J Prosthodont. 2016; 29:369–371.
15. Guess PC, Zhang Y, Thompson VP. Effect of veneering techniques on damage and reliability of Y-TZP trilayers. Eur J Esthet Dent. 2009; 4:262–276.
16. Ambre MJ, Aschan F, Vult von Steyern P. Fracture strength of yttria-stabilized zirconium-dioxide (Y-TZP) fixed dental prostheses (FPDs) with different abutment core thicknesses and connector dimensions. J Prosthodont. 2013; 22:377–382.
17. Wimmer T, Erdelt KJ, Eichberger M, Roos M, Edelhoff D, Stawarczyk B. Influence of abutment model materials on the fracture loads of three-unit fixed dental prostheses. Dent Mater J. 2014; 33:717–724.
18. Nawafleh N, Hatamleh M, Elshiyab S, Mack F. Lithium disilicate restorations fatigue testing parameters: A systematic review. J Prosthodont. 2016; 25:116–126.
19. Kelly JR. Clinically relevant approach to failure testing of all-ceramic restorations. J Prosthet Dent. 1999; 81:652–661.
20. Wiskott HW, Nicholls JI, Belser UC. Stress fatigue: basic principles and prosthodontic implications. Int J Prosthodont. 1995; 8:105–116.
21. Zhang L, Wang Z, Chen J, Zhou W, Zhang S. Probabilistic fatigue analysis of all-ceramic crowns based on the finite element method. J Biomech. 2010; 43:2321–2326.
22. Beuer F, Steff B, Naumann M, Sorensen JA. Load-bearing capacity of all-ceramic three-unit fixed partial dentures with different computer-aided design (CAD)/computer-aided manufacturing (CAM) fabricated framework materials. Eur J Oral Sci. 2008; 116:381–386.
23. McLaren EA, Giordano RA. Zirconia-based ceramics: Material properties, esthetics, and layering techniques of a new veneering porcelain, VM9. Quintessence Dent Technol. 2005; 28:99–112.
24. Preis V, Weiser F, Handel G, Rosentritt M. Wear performance of monolithic dental ceramics with different surface treatments. Quintessence Int. 2013; 44:393–405.
25. DeLong R. Intra-oral restorative materials wear: rethinking the current approaches: how to measure wear. Dent Mater. 2006; 22:702–711.
26. Woda A, Mishellany A, Peyron MA. The regulation of masticatory function and food bolus formation. J Oral Rehabil. 2006; 33:840–849.
27. Rees JS. An investigation into the importance of the periodontal ligament and alveolar bone as supporting structures in finite element studies. J Oral Rehabil. 2001; 28:425–432.
28. Cho L, Song H, Koak J, Heo S. Marginal accuracy and fracture strength of ceromer/fiber-reinforced composite crowns: effect of variations in preparation design. J Prosthet Dent. 2002; 88:388–395.
29. Rosentritt M, Naumann M, Hahnel S, Handel G, Reill M. Evaluation of tooth analogs and type of restoration on the fracture resistance of post and core restored incisors. J Biomed Mater Res B Appl Biomater. 2009; 91:272–276.
30. Tinschert J, Natt G, Mautsch W, Augthun M, Spiekermann H. Fracture resistance of lithium disilicate-, alumina-, and zirconia-based three-unit fixed partial dentures: a laboratory study. Int J Prosthodont. 2001; 14:231–238.
31. Scherrer SS, de Rijk WG. The fracture resistance of all-ceramic crowns on supporting structures with different elastic moduli. Int J Prosthodont. 1993; 6:462–467.
32. Potiket N, Chiche G, Finger IM. In vitro fracture strength of teeth restored with different all-ceramic crown systems. J Prosthet Dent. 2004; 92:491–495.
33. Bindl A, Lüthy H, Mörmann WH. Strength and fracture pattern of monolithic CAD/CAM-generated posterior crowns. Dent Mater. 2006; 22:29–36.
34. Rosentritt M, Behr M, Gebhard R, Handel G. Influence of stress simulation parameters on the fracture strength of all-ceramic fixed-partial dentures. Dent Mater. 2006; 22:176–182.
35. Sorensen JA, Choi C, Fanuscu MI, Mito WT. IPS Empress crown system: three-year clinical trial results. J Calif Dent Assoc. 1998; 26:130–136.
36. Clausen JO, Abou Tara M, Kern M. Dynamic fatigue and fracture resistance of non-retentive all-ceramic full-coverage molar restorations. Influence of ceramic material and preparation design. Dent Mater. 2010; 26:533–538.
37. Della Bona A, Mecholsky JJ Jr, Anusavice KJ. Fracture behavior of lithia disilicate- and leucite-based ceramics. Dent Mater. 2004; 20:956–962.
38. Apel E, Deubener J, Bernard A, Höland M, Müller R, Kappert H, Rheinberger V, Höland W. Phenomena and mechanisms of crack propagation in glass-ceramics. J Mech Behav Biomed Mater. 2008; 1:313–325.
39. Rodríguez V, Castillo-Oyagüe R, López-Suárez C, Gonzalo E, Peláez J, Suárez-García MJ. Fracture load before and after veneering zirconia posterior fixed dental prostheses. J Prosthodont. 2016; 25:550–556.
40. Garvie RC, Hannink RH, Passcoe RT. Ceramic Steel? Nature. 1975; 258:703–704.
41. Luthardt RG, Sandkuhl O, Reitz B. Zirconia-TZP and alumina-advanced technologies for the manufacturing of single crowns. Eur J Prosthodont Restor Dent. 1999; 7:113–119.
42. Weyhrauch M, Igiel C, Scheller H, Weibrich G, Lehmann KM. Fracture strength of monolithic all-ceramic crowns on titanium implant abutments. Int J Oral Maxillofac Implants. 2016; 31:304–309.
43. Belli R, Petschelt A, Hofner B, Hajtó J, Scherrer SS, Lohbauer U. Fracture rates and lifetime estimations of CAD/CAM all-ceramic restorations. J Dent Res. 2016; 95:67–73.
44. Guess PC, Zavanelli RA, Silva NR, Bonfante EA, Coelho PG, Thompson VP. Monolithic CAD/CAM lithium disilicate versus veneered Y-TZP crowns: comparison of failure modes and reliability after fatigue. Int J Prosthodont. 2010; 23:434–442.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download