Abstract
PURPOSE
To investigate the effect of non-thermal atmospheric pressure plasma (NTAPP) treatment on shear bond strength (SBS) between resin cement and colored zirconia made with metal chlorides.
MATERIALS AND METHODS
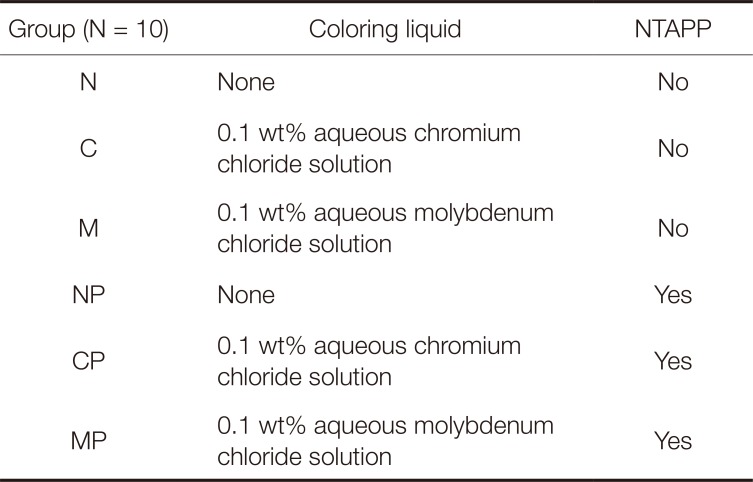
60 zirconia specimens were divided into 3 groups using coloring liquid. Each group was divided again into 2 sub-groups using plasma treatment; the experimental group was treated with plasma, and the control group was untreated. The sub-groups were: N (non-colored), C (0.1 wt% aqueous chromium chloride solution), M (0.1 wt% aqueous molybdenum chloride solution), NP (non-colored with plasma), CP (0.1 wt% aqueous chromium chloride solution with plasma), and MP (0.1 wt% aqueous molybdenum chloride solution with plasma). Composite resin cylinders were bonded to zirconia specimens with MDP-based resin cement, and SBS was measured using a universal testing machine. All data was analyzed statistically using a 2-way ANOVA test and a Tukey test.
Zirconia is one of the most popular ceramic materials currently being used in dentistry. Its opaque natural color makes it ideal for treating discolored teeth, and it is highly resistant to fracture. In addition, zirconia generates less aggressive wear on the opposing tooth, when compared to feldspathic porcelain.1 However, it is difficult to fabricate aesthetic restorations with zirconia because of its intrinsic color, and as such tinted zirconia is now being developed to compensate for this limitation. One such method involves mixing in a colorant with the zirconia powder during the composition process;2 the other produces the desired color tone by immersing partially sintered zirconia into a solution that contains minerals, such as chrome or molybdenum, which have been found to produce tones within the color range of natural teeth.345 Kim et al.6 revealed that coloring zirconium with a 0.1 wt% aqueous solution of molybdenum chloride resulted in significantly improved aesthetics and function when compared with conventional non-colored zirconia.
Bonding with resin cement is problematic because zirconia does not contain silica,67 therefore, various methods have been developed to increase the bond strength between these two materials. Although air abrasion using alumina particles has been shown to improve mechanical bond strength,89 it can also cause cracking of the zirconia surface and loss of zirconia particles.10 For this reason, novel methods that do not lead to mechanical changes to the zirconia surface have been sought. Plasma is one of the new treatments and is currently being tested as a dental biomaterial for direct/indirect application in the oral cavity.111213 To improve bond strength, plasma treatment increases wettability and surface adhesion without changing the material itself, producing a thin film of polymer with a functional end-group by activating and deactivating steps.1415 Derand et al.12 reported that plasma treatment increased the bonding strength between resin cement and zirconia, and Canullo et al.13 reported that argon plasma cleaning improved the wettability of the zirconia surface and increased the bond strength of methacryloyloxydecyl dihydrogen phosphate (MDP)-based resin cement. In contrast, studies involving colored zirconia are still lacking, although a number of experiments have been conducted that used plasma for improving bonding strength between non-colored zirconia and resin cement.
In this study, we have evaluated the effect of non-thermal atmospheric pressure plasma (NTAPP) treatment on the shear bond strength (SBS) of fabricated colored zirconia using a metal chloride solution and resin cement. The first null hypothesis is that NTAPP has no effect on the SBS between colored zirconia and resin cement. The second null hypothesis is that there is no significant difference in the SBS between the NTAPP-treated colored zirconia and the NTAPP-treated non-colored zirconia.
Table 1 describes the specimens used in this study. Among 60 zirconia specimens, the control group consisted of non-colored zirconia specimens, whereas the experimental group included colored zirconia specimens by commercial 5.4 wt% yttrium-stabilized tetragonal zirconia powder (KZ-3YF type AC, KCM, Nagoya, Japan)5 immersed in a 0.1 wt% aqueous chromium chloride solution or a 0.1 wt% aqueous molybdenum chloride solution. Fig. 1 shows the representative zirconia specimens used in this experiment.
Zirconia powder was placed in a cylindrical metal mold and was then pressed and transformed into 20 mm diameter, 2.75 mm thick zirconia specimens. After heating the specimens in a furnace (Ovmat 2009, Manfredi, Italy), increasing the temperature from 25℃ to 1040℃ at a rate of 1℃/min, pre-sintering was completed by cooling for 90 minutes. One-third of the pre-sintered zirconia specimens (n = 20) underwent final sintering (Kavo Therm, Kavo, Biberach, Germany) and constituted the non-colored zirconia control group (group N). The remaining 40 specimens were put in to a furnace for coloring, with the temperature increased from room temperature to 500℃ at a rate of 10℃/min. The experimental specimens were held for 3 minutes at this temperature before the temperature was increased to 600℃ for 5 minutes, after which they were left to cool at room temperature for 10 minutes. These specimens were immersed for 3 minutes in 2 solutions, 0.1 wt% aqueous chromium chloride solution (group C) and 0.1 wt% aqueous molybdenum chloride solution (group M), and were then dried and sintered for 20 minutes at a temperature of up to 1000℃. After raising the temperature to 1450℃ and maintaining it for 120 minutes, we allowed the specimens to reach room temperature. The sintered zirconia specimens had a final diameter of 15 mm and a thickness of 2 mm and were polished under water using 600 SiC polishing paper on a polishing instrument (LaboPol-5, Struers, Ballerup, Denmark).6
An NTAPP generator (Model PGS-200, Expantech, Suwon, Korea) was used to treat the zirconia surfaces. Plasma gas (100% argon) was applied at a rate of 10 standard liters per minute (slm) while each specimen was rotated to the left at 300 rpm, evenly exposed for 30 seconds. The zirconia groups with NTAPP were indicated as groups NP, CP and MP (Table 1). Table 2 shows the experimental conditions.
A composite resin (Filtek Z250, 3M ESPE, St. Paul, MN, USA) was used to produce a cylindrical metal mold 6 mm thick and 3 mm in diameter, using an LED polymerizing device (Elipar FreeLight 2, 3M ESPE, St. Paul, MN, USA). The zirconia specimens and composite resin cylinders were bonded with an MDP-based resin cement (PANAVIA F 2.0, Kuraray Medical, Osaka, Japan) (Fig. 2A), and SBS was measured using a universal testing machine (RB Model 301, Unitech M, R&B Co., Ltd., Daejeon, Korea) (Fig. 2B).
Each specimen was attached to a jig and loaded against a composite resin cylinder at a crosshead speed of 0.5 mm/min. SBS was calculated after measuring the maximum load at which the composite resin cylinder separated from the zirconia.6 The fractured surfaces were observed by field emission scanning electron microscopy (FE-SEM: S-4700, Hitachi Ltd.) at ×180 magnifications, operating at 15 keV.
SBS was evaluated statistically with SPSS software version 21 for Windows (IBM, Chicago, IL, USA). Two-way ANOVA and Tukey's honest significant difference (HSD) tests were used for post hoc comparisons based on the coloring liquid used and whether or not the specimens were treated with NTAPP. To investigate the effect of NTAPP on each specimen, an independent t-test was used. A P value of less than 0.05 was considered to be statistically significant.
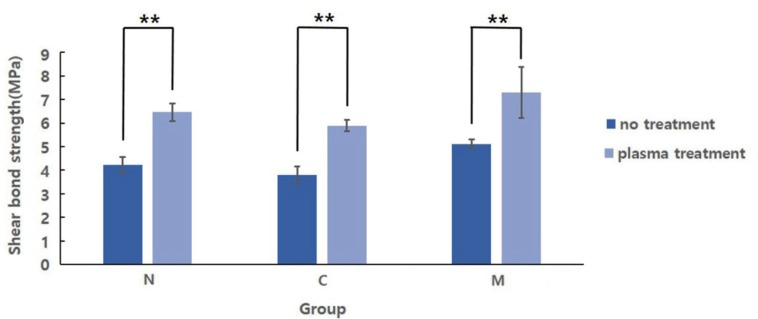
After statistical analysis with two-way ANOVA, both NTAPP and coloring were found to have a significant impact on SBS (P < .001); however, no significant interaction was observed (P > .05) (Table 3). The mean (± SD) MPa values for N group, C group, and M group were 4.24 ± 0.334, 3.79 ± 0.367, and 5.12 ± 0.181, respectively. One-way ANOVA confirmed that the SBS for each group differed significantly (P < .001). The post-hoc Tukey HSD test confirmed a significant difference between chromium group (P < .05) and molybdenum group (P < .001) compared with non-colored group (Fig. 3). The SBS values for NP group, CP group, and MP group were 6.46 ± 0.372, 5.89 ± 0.237, and 7.29 ± 1.082 MPa, respectively, and additional statistical analysis indicated significant differences among the specimens (P < .001). Results of Tukey's HSD test showed no significant difference between non-colored with plasma group and chromium with plasma group, whereas molybdenum with plasma group exhibited a significantly higher SBS (Fig. 4). Independent t-tests, to compare SBS values with respect to NTAPP in each group, confirmed a significant increase in the average SBS for all groups (P < .001) (Fig. 5). Fig. 6 shows representative SEM images of fractured surface. All groups showed adhesive failure mode.
The aim of this study was to evaluate the effect of NTAPP treatment on SBS between colored zirconia and a type of MDP-based resin cement. The results showed that SBS increased significantly among the specimens treated with NTAPP. Thus, the first null hypothesis could be rejected. Moreover, the NTAPP-treated zirconia specimens showed statistically significant difference in SBS, independent of whether they had been colored or not. However, SBS on NTAPP-treated colored zirconia was higher than non-colored zirconia, and so the second null hypothesis was rejected.
Cales16 reported that specific coloring of zirconia could be achieved by adding different metal oxides. On the other hand, Oh et al.5 used metal chloride to color zirconia by means of the liquid immersion method. When adding metal chloride to color the zirconia, an oxygen vacancy is produced, replacing zirconium with a transition metal ion. Oxide is formed when the disassembled oxygen atom combines with the transition metal.417 Oh et al.5 produced chloride-colored zirconia that showed improved mechanical properties and biocompatibility. From among the various metal chlorides, we chose 0.1 wt% aqueous chromium chloride solution and 0.1 wt% aqueous molybdenum chloride solution, because they can both simulate natural tooth shades but do not cause cytotoxicity.45 In a previous study,6 zirconia specimens that were immersed in aqueous molybdenum chloride solution showed significantly higher SBS values, compared with other specimens, as aqueous molybdenum chloride solution changed the granular boundary of zirconia unevenly. This phenomenon that changed zirconia particle size and morphology enhanced the abrasion resistance, tensile strength, stability, and bond strength.181920
Dental cement plays an important role in restoration longevity by improving the retention of the restoration. A previous study18 reported that the bond strength of zirconia depended upon the type of cement used, regardless of the surface treatment. Many researchers have found that the use of Panavia F 2.0 with MDP-based resin cement can increase SBS with zirconia;192021 and for this reason, we used Panavia F 2.0 in our study.
As zirconia lacks a silica component, it is generally not subjected to acid etching, which is performed prior to resin bonding. In contrast to feldspathic porcelain, zirconia bonding is more difficult, and many methods have been attempted to improve it. However, our study was not an attempt to increase mechanical bonding to determine the distinct effects of plasma treatment.
Plasma treatment produces a chemically altered surface on certain materials to facilitate the formation of peroxide radicals (R-O-O).24252627 The functional groups on the altered zirconia surface can combine with phosphate (C=O) in the resin cement, thus increasing the bond strength between the cement and the zirconia.28 Also, the plasma treatment enhances chemical bonding by introducing a secondary force, according to the Van der Waals equation.29 In addition, in the case of plasma, a previous study30 indicated that the decrease in bonding strength, as a result of polluted organic material in zirconia, can be overcome by increasing the plasma's cleansing potential by decreasing the absorbed hydrocarbon through severing the bond between C-H and C-C.
Although SBS was higher with NTAPP treatment than without it, our subsequent analysis of the failure modes under FE-SEM (S-4700, Hitachi Ltd., Tokyo, Japan) showed that all specimens were presented adhesive failure mode. Our results here suggest that this phenomenon warrants additional study.
Many studies in which zirconia is being treated with NTAPP are underway, however the specific application of NTAPP to colored zirconia using metal chloride still requires investigation. We confirmed that the physical and chemical features of colored zirconia remain stable with such treatment, since there was no significant difference in the response of non-colored zirconia when compared to the colored zirconia. In particular, colored zirconia immersed in 0.1 wt% aqueous molybdenum chloride solution can potentially be utilized in many fields, because SBS is greatly increased by the application of NTAPP. Meanwhile, the NTAPP-treated colored zirconia immersed in 0.1 wt% aqueous molybdenum chloride solution showed the highest standard deviation, which means the mechanism of this phenomenon remains to be determined. Additionally, for the best potential clinical applications, future experiments to assess the type of gas used for the plasma treatment, optimum processing times, and combinations with other surface treatments, should be undertaken.
References
1. Preis V, Behr M, Kolbeck C, Hahnel S, Handel G, Rosentritt M. Wear performance of substructure ceramics and veneering porcelains. Dent Mater. 2011; 27:796–804. PMID: 21524788.

2. Aboushelib MN, de Jager N, Kleverlaan CJ, Feilzer AJ. Microtensile bond strength of different components of core veneered all-ceramic restorations. Dent Mater. 2005; 21:984–991. PMID: 16085302.

3. Heffernan MJ, Aquilino SA, Diaz-Arnold AM, Haselton DR, Stanford CM, Vargas MA. Relative translucency of six all-ceramic systems. Part II: core and veneer materials. J Prosthet Dent. 2002; 88:10–15. PMID: 12239473.

4. Yuan JC, Brewer JD, Monaco EA Jr, Davis EL. Defining a natural tooth color space based on a 3-dimensional shade system. J Prosthet Dent. 2007; 98:110–119. PMID: 17692592.

5. Oh GJ, Lee KM, Lee DJ, Lim HP, Yun KD, Ban JS, Lee KK, Fisher JG, Park SW. Effect of metal chloride solutions on coloration and biaxial flexural strength of yttria-stabilized zirconia. Met Mater Int. 2012; 18:805–812.

6. Kim GH, Park SW, Lee K, Oh GJ, Lim HP. Shear bond strength between resin cement and colored zirconia made with metal chlorides. J Prosthet Dent. 2015; 113:603–608. PMID: 25819355.

7. Behr M, Proff P, Kolbeck C, Langrieger S, Kunze J, Handel G, Rosentritt M. The bond strength of the resin-to-zirconia interface using different bonding concepts. J Mech Behav Biomed Mater. 2011; 4:2–8. PMID: 21094475.

8. Chang JC, Powers JM, Hart D. Bond strength of composite to alloy treated with bonding systems. J Prosthodont. 1993; 2:110–114. PMID: 8242163.

9. Blatz MB, Sadan A, Martin J, Lang B. In vitro evaluation of shear bond strengths of resin to densely-sintered high-purity zirconium-oxide ceramic after long-term storage and thermal cycling. J Prosthet Dent. 2004; 91:356–362. PMID: 15116037.

10. Phark JH, Duarte S Jr, Blatz M, Sadan A. An in vitro evaluation of the long-term resin bond to a new densely sintered high-purity zirconium-oxide ceramic surface. J Prosthet Dent. 2009; 101:29–38. PMID: 19105989.

11. von Woedtke T, Metelmann HR, Weltmann KD. Clinical plasma medicine: state and perspectives of in vivo application of cold atmospheric plasma. Contributions Plasma Phys. 2014; 54:104–117.
12. Derand T, Molin M, Kvam K. Bond strength of composite luting cement to zirconia ceramic surfaces. Dent Mater. 2005; 21:1158–1162. PMID: 16005508.

13. Canullo L, Micarelli C, Bettazzoni L, Koçi B, Baldissara P. Zirconia-composite bonding after plasma of argon treatment. Int J Prosthodont. 2014; 27:267–269. PMID: 24905269.

14. Shim JW, Lee K, Jeong MJ, Jung SC, Kim BH. Hyaluronic acid immobilization on the poly-allylamine coated nano-network TiO2 surface. J Nanosci Nanotechnol. 2011; 11:7343–7346. PMID: 22103192.

15. Rammelt S, Illert T, Bierbaum S, Scharnweber D, Zwipp H, Schneiders W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials. 2006; 27:5561–5571. PMID: 16879866.

16. Cales B. Colored zirconia ceramics for dental applications. Bioceram. 1998; 11:591–594.
17. Shah K, Holloway JA, Denry IL. Effect of coloring with various metal oxides on the microstructure, color, and flexural strength of 3Y-TZP. J Biomed Mater Res B Appl Biomater. 2008; 87:329–337. PMID: 18433010.

18. Ueno T, Kumagai T, Hirota K, Saitoh Y, Kojima I. The characteristic of metal adhesion primer GC Metal Primer II. J Dent Res. 1997; 76:312.
19. Beuer F, Erdelt K, Schweiger J, Eichberger M. Flexural strength of coloured and aged zirconia. J Dent Res. 2004; 83:942–954.
20. Bartolomé JF, Díaz M, Moya JS. Influence of the metal particle size on the crack growth resistance in mullite-molybdenum composites. J Am Ceram Soc. 2002; 85:2778–2784.

21. Sbaizero O, Roitti S, Pezzotti G. R-curve behavior of alumina toughened with molybdenum and zirconia particles. Mat Sci Eng A. 2003; 359:297–302.

22. Khan AA, Labbe JC. Aluminium nitride–molybdenum ceramic matrix composites: influence of molybdenum concentration on the mechanical properties. J Mater Sci. 1997; 32:3829–3833.
23. Ozcan M, Vallittu PK. Effect of surface conditioning methods on the bond strength of luting cement to ceramics. Dent Mater. 2003; 19:725–731. PMID: 14511730.
24. Kern M, Wegner SM. Bonding to zirconia ceramic: adhesion methods and their durability. Dent Mater. 1998; 14:64–71. PMID: 9972153.

25. Wegner SM, Gerdes W, Kern M. Effect of different artificial aging conditions on ceramic-composite bond strength. Int J Prosthodont. 2002; 15:267–272. PMID: 12066490.
26. Lüthy H, Loeffel O, Hammerle CH. Effect of thermocycling on bond strength of luting cements to zirconia ceramic. Dent Mater. 2006; 22:195–200. PMID: 16143382.
27. Silva NR, Coelho PG, Valverde GB, Becker K, Ihrke R, Quade A, Thompson VP. Surface characterization of Ti and Y-TZP following non-thermal plasma exposure. J Biomed Mater Res B Appl Biomater. 2011; 99:199–206. PMID: 21714084.

28. Kim JH, Lee MA, Han GJ, Cho BH. Plasma in dentistry: a review of basic concepts and applications in dentistry. Acta Odontol Scand. 2014; 72:1–12. PMID: 24354926.

29. Zhang W, Chu PK, Ji J, Zhang Y, Liu X, Fu RK, Ha PC, Yan Q. Plasma surface modification of poly vinyl chloride for improvement of antibacterial properties. Biomaterials. 2006; 27:44–51. PMID: 16005957.

30. Yavirach P, Chaijareenont P, Boonyawan D, Pattamapun K, Tunma S, Takahashi H, Arksornnukit M. Effects of plasma treatment on the shear bond strength between fiber-reinforced composite posts and resin composite for core buildup. Dent Mater J. 2009; 28:686–692. PMID: 20019419.

31. Yoshida K, Tsuo Y, Atsuta M. Bonding of dual-cured resin cement to zirconia ceramic using phosphate acid ester monomer and zirconate coupler. J Biomed Mater Res B Appl Biomater. 2006; 77:28–33. PMID: 16193486.

32. Valverde GB, Coelho PG, Janal MN, Lorenzoni FC, Carvalho RM, Thompson VP, Weltemann KD, Silva NR. Surface characterisation and bonding of Y-TZP following non-thermal plasma treatment. J Dent. 2013; 41:51–59. PMID: 23044388.

33. Amaral R, Ozcan M, Bottino MA, Valandro LF. Microtensile bond strength of a resin cement to glass infiltrated zirconia-reinforced ceramic: the effect of surface conditioning. Dent Mater. 2006; 22:283–290. PMID: 16039705.

Fig. 1
Experimental groups. (A) Uncolored zirconia, (B) 0.1 wt% chromium coloring liquid, (C) 0.1 wt% molybdenum coloring liquid.
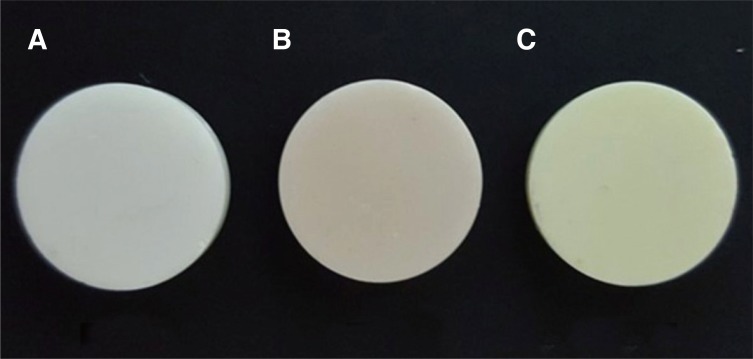
Fig. 2
Process of shear bond strength test. (A) Composite resin cylinder cemented on zirconia disc, (B) Universal testing machine.
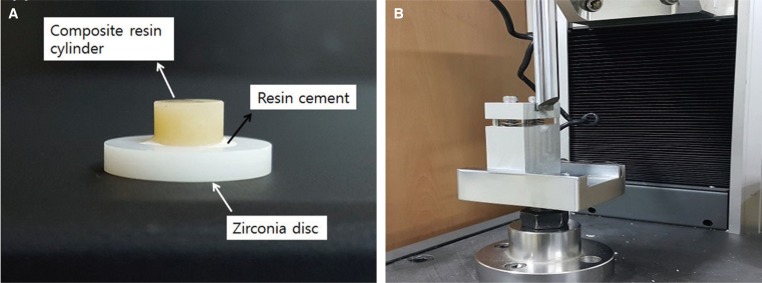
Fig. 3
Result of Tukey HSD test for shear bond strength before plasma treatment (*: significant at P < .05, **: significant at P < .001).

Fig. 4
Result of Tukey HSD test for shear bond strength after plasma treatment (*: significant at P < .05).
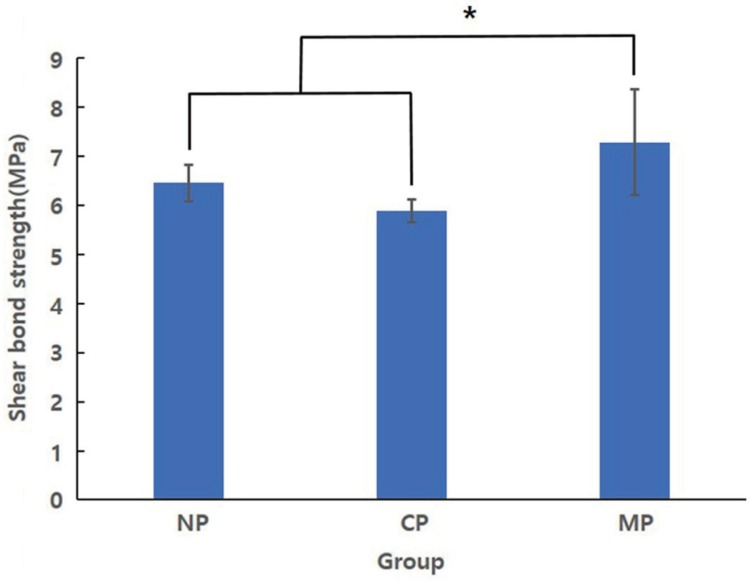
Fig. 6
SEM representative image of specimen fractured surfaces (×180 magnification). (A) Non-colored, (B) Non-colored with plasma, (C) Chromium colored, (D) Chromium colored with plasma, (E) Molybdenum colored, (F) Molybdenum colored with plasma (R is composite resin, Z is zirconia specimen).
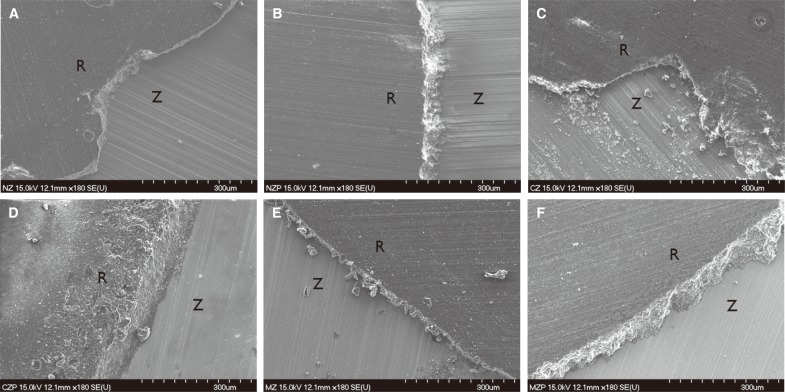
Table 1
Experimental groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download