INTRODUCTION
Nowadays, implantology forms an integral part of dentists' therapeutic arsenal for treating single or multiple missing teeth. Implants are frequently considered to be, and justly so, the optimal solution for reconstructing one or more missing teeth, from both a functional and a psychological point of view.
1
During the last few years, there has been a remarkable progress in the field of implantology. Initially used solely for denture stabilization, implant dentistry now allows fixed implant-supported dentures for single or multiple missing teeth.
2 Improvements of surgical methods and the progress in making better osseointegration result in a very high success rate today.
2
These particular advances and the technical developments in implantology have created a situation in which the challenges we face are not only adequate osseointegration of the implant, this having already been well mastered, but achieving esthetically better results.
Faced with increasingly demanding patients, practitioners are forced to push back the limits of biomimicry, in order to be able to satisfy them.
After prosthetic restoration of the anterior teeth, the natural appearance of the peri-implant gingiva will be seriously affected by the implant's volume, shape, and color.
3 Surgical expertise during the actual procedure, good positioning of the implant with regard to the three dimensional space,
45 as well as fine handling of the soft tissues, potentially preceded by increasing the amount of soft tissue,
6 will be the primary factors for obtaining a harmonious and natural prosthesis-gingiva interface.
The prosthetic phase will play an equally crucial role. During soft tissue healing, the prosthesis will guide the gingiva, in order to obtain a suitable emerging profile. But what about the choice of implant abutment? Histological factors, such as the intensity of melanogenesis, degree of keratinization, and even capillary density, affect color,
7 and it may also be influenced by the restoration material
8 and in this particular case by the implant abutment.
39
The implant abutment will ensure the transition between the implant and the prosthetic tooth. It may be:
- Prefabricated core build-up, modified by reaming intraorally or in the laboratory
- An individualized abutment, adapted to the gingival margin using a digital procedure (e.g. Procera)
- Castable abutment, but with a machined base for a perfect fit on the implant replicate (e.g. UCLA10 abutment).
Nowadays there are a variety of materials used for making implant abutments,
10 and the four main ones will be included in this study. They may be classified into two categories:
-
1) Metal abutments
-
2) Ceramic abutments
- Zirconium dioxide
- Aluminium oxide
The purpose of this study is to evaluate the different types of materials used for making implant abutments, by means of an in vitro study and a review of the literature, in order to identify the indications for a better choice of an implant-supported restoration in the anterior section.
MATERIALS AND METHODS
This study tries to demonstrate the aesthetic properties of materials through an
in vitro study inspired by Jung et al.'s 2007 protocol.
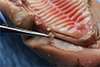
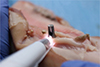
3 The study was conducted on 8 different pig maxillae (
Fig. 1). These pigs had been slaughtered for food purposes, in accordance with the World Organization for Animal Health standards. Therefore, this study is considered to be an animal study and does not require submission to the FUB-Erasmus Hospital-Faculty Ethics Committee although this committee had been consulted in advance.
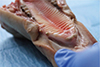
Pig gingiva share many similarities with the human keratinized mucosa. In order to be able to simulate different levels of gingival thickness, flaps of 1 mm connective tissue in thickness were removed, from the anterior sector (
Fig. 2).
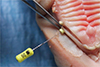
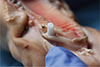
The final thickness of the different sites was measured using an endodontic file (
Fig. 3). To minimize artifacts, the different pieces were moistened with saline solution before being superimposed. On each of the sites, 5 implant abutments were tested in a random order:
1) Abutment made of titanium dioxide (Nobel Biocare, Kloten, Switzerland)
2) Abutment made of zirconium dioxide, Standard BO shade (Nobel Biocare)
3) Abutment made of zirconium dioxide, Light BI shade (Nobel Biocare)
4) Abutment made of zirconium dioxide, Intense A 3.5 shade (Nobel Biocare)
5) Abutment made of aluminium oxide
In order to objectively evaluate color, a VITA Easyshade Advance spectrophotometer (Vita Zahnfabrik, Bad Sackingen, Germany) was used. Designed for determining tooth color, the device has a VITA SYSTEM 3D- MASTER mode, which also provides the parameters defined by the ICI: L for luminosity, a for absorbance in the red-green range, and b for absorbance in the yellow-blue range.
The site chosen was the superior anterior maxilla. After a full-thickness intrasulcular incision was made with a n° 15 blade, the mucoperiosteal flap was removed using a rugine.
For each operative site, the thickness was measured using an endodontic file, and the first spectrophotometric measurement was recorded by gently placing the spectrophotometer in contact with the gingiva. This site of measurement served as the control site. In each site, we inserted a replica implant (Nobel Biocare) with an internal hexagonal connection.
In a random order, the abutments were placed at the site and the color was analysed using the spectrophotometer. Depending on the thickness measured at the abutment neck, one or more 1 mm-thick flaps were superimposed, in order to simulate a gingival biotype, either thin (2 mm) or thick (3 mm). The 5 abutments were thus tested for each of the two thicknesses (
Fig. 4 and
Fig. 5). The same manipulation was repeated on the 8 superior maxillae by the same surgeon (
Fig. 6). Thus, each abutment (5) was tested twice (2 mm and 3 mm) on 8 pig maxillae.
In order to determine the degree of color variation caused by each abutment, the values of the ICI parameters L, a, and b, measured at the control site were subtracted from those at the test site, and the difference in color (ΔE) produced by each abutment was calculated using the following equation: ΔE = (ΔL
2 + Δa
2 + Δb
2)
1/2.
11 The resulting data was analyzed using the SPSS program, version 22.
The review of the literature for this dissertation was conducted starting with a search on PubMed and the Science Direct Wiley Online Library database, and also using the Google Scholar search engine. The following keywords were used:
‘Abutment’, ‘esthetic abutment’, ‘gingival color abutment’, ‘titanium abutment’, ‘zirconium abutment’, ‘alumina abutment’, ‘gold abutment’, and ‘ceramic abutment’.
For results concerning the aesthetic aspects of abutments, studies done in the years between 2000 and 2014 and, of these, the ones exclusively based on spectrophotometric analysis were chosen, thus excluding the studies of the ‘Pink Aesthetic Score’ type 11.
12 These studies were classified and compared using the following parameters: ‘study type’, ‘sample size’, ‘variables studied’, ‘results’, ‘gingival thickness’ and ‘position relative to the marginal limit’ (
Table 1).
DISCUSSION
Our in vitro study proves that implant abutment placement causes color change to the overlying gingiva, the change having little to do with the type of material from which the abutment is made. This color change, which can be unsightly and compromise the aesthetic success of implant prosthetic treatment, is variable depending on type of material and gingival thickness.
In our study, when gingival thickness was 2 mm, only an abutment made of zirconium dioxide, Standard shade, did not result in a visible change of color. When we simulated a 3 mm thickness gingival biotype, all of the color changes were reduced, except in the case of zirconium, which already had a value below the visibility threshold. Finally, only aluminium oxide resulted in a visible color change each time.
The small sample size and the
in vitro nature of the study imply that these results should be interpreted with caution. In the scientific literature, many cases of a greyish peri-implant discolouration of the gingiva have been previously reported (
Table 1).
13 After many case report publications, these discolourations were objectively measured
in vivo, for the first time, by Jung et al.
9 in 2008, using spectrophotometric measurements in a randomized, prospective, clinical study. They showed that the placement of a titanium abutment caused visible gingival discolouration that was unsightly and inharmonious with the adjacent tooth's gingiva.
Numerous materials are used to make implant abutments, and these can be classified into two main categories:
Metal abutments can be made of titanium or gold. These abutments were the first to be used. Titanium is a rigid material that breaks when submitted to forces. Titanium abutments have ease of use and they have excellent biocompatibility features
14 combined with a minimal risk of corrosion when coming into contact with the implant.
15 This is also the material where most clinical experience has been gained.
The majority of abutments made of a gold alloy are UCLA type abutments. Gold is a material that suffers from distortion when submitted to forces. These abutments are made up of a machined golden part, combined with a plastic sheath cast onto an alloy of precious metals (a gold-palladium alloy).
10 This method will allow abutments to be made so they are adapted to the clinical situation, in relation to both gingival contour and angulation. However, according to the study by Andersson et al.,
16 even though gingival discolourations around golden abutments appeared to be significantly lower than discolourations around a titanium abutment, they still remained visible, when compared to the natural tooth.
For Jung et al.,
9 this type of abutment also caused a peri-implant gingival discolouration that was significant and visible.
In order to reduce unsightly gingival discolouration, various kinds of ceramic abutments have been designed: aluminium oxide and zirconium oxide abutments.
Presented in a prospective, randomized clinical study by Jung et al.,
9 abutments made of aluminium oxide exhibited excellent optical properties, causing significantly weaker variation of gingival color, compared to titanium or golden abutments. They were made up of more than 99.5% aluminium oxide with traces of magnesium oxide, calcium and alkali metals. Unfortunately, with a flexion resistance of only 520 MPa, abutments made of aluminium oxide have shown the limits of their mechanical properties and numerous cases of fracture have been recorded.
16
Zirconium oxide abutments are made from tetragonal polycrystalline zirconium, stabilized with yttrium oxide, is, due to its excellent mechanical properties being both resistant and of fine texture, the ceramic is the material of choice, especially for frame design in fixed prosthesis.
17 In vitro, its mechanical resistance is twice that of aluminium oxide, with a flexion resistance of 1120 MPa.
18 In an
in vivo study, Rimondini et al.
19 have shown that bacterial adhesion to zirconium oxide abutments was significantly lower than that to titanium oxide abutments.
Regarding optical properties, the studies were not entirely in agreement, but the authors
392021 agreed on the fact that zirconium oxide abutments caused a color change to the peri-implant gingiva. This change was significantly lower than the change caused by either titanium or gold, but it remained visible in the
in vivo studies.
Subsequently, laboratories offered a multitude of shades for zirconia abutments, incorporating pigmented powders during the material's preparation, as was the case for the zirconia light BI shade and intense A 3.5 shade abutments used in our study.
Even though there were, indeed, multiple studies regarding discolouration caused by implant abutments, the results did not always overlap. However, these differences might be explained, in part, by multiple factors that are the evaluation of gingival color, the differences at the tested sites, grafted sites, the measurement of soft tissue thickness, the method for measuring gingival thickness and the Correlation between gingival thickness and optimal choice of abutment.
In order to evaluate the color of each site in an objective and replicable manner, the values of the following parameters were recorded: Luminosity (L), absorbance in the red-green range (a) and absorbance in the yellow-blue range (b). These values were either directly recorded at the site using a spectrophotometer, or they were collected after digital analysis of site pictures. Therefore, there was already a first difference in the method of data acquisition for color-related data. This data has allowed us to study color variation between different sites, in accordance with the recommendations of the International Commission on Luminosity (1976): ΔE = (ΔL2 + Δa2 + Δb2)1/2.
Meanwhile, in order for it to be pertinent, the ΔE value must be compared to the threshold perceived by the human eye.
Under laboratory conditions,
22 the human eye can distinguish a color variation equal to ΔE = 1. However, inside the oral cavity, this capability decreases and the variation has to be ΔE > 3.7 to be clinically discernible.
23
In order to study color variation caused by the abutment, compared to a control site, only two studies.
2021 have followed a cross-over protocol, alternating different abutments on the same test site in a random order. In this event, the protocol may bring the risk that the results might be biased, either due to pre-existing heterogeneity of the gingival color
24 between two adjacent sites, heterogeneity that may reach a value of ΔE = 2.7, or gingival color differences between two groups of patients to be excluded.
13
Certain studies
925 included a group of patients who had benefited from a connective tissue autograft on the test site. It cannot be neglected that this tissue augmentation may cause residual variation to the gingival color.
Not all studies on gingival peri-implant discolouration measure gingival thickness. It does, however, seem legitimate to think that a particular thickness might affect (or not) the diffusion of discolouration caused by implant abutments (cf. 6).
Out of 10 studies comparing different materials using a spectrophotometer, only 7 included gingival thickness as a parameter. In our study, gingival thickness has played a significant role and, when it was 3 mm, the changes caused by titanium and zirconia abutments fell under the visibility threshold.
The thickness was measured either directly at the site level using an endodontic file, or indirectly, on a digital photograph or on the laboratory replica model. A measurement bias may therefore be present and, at this point, there is no published study comparing the efficacy of these methods for measuring soft tissue thickness.
In an
in vitro study,
3 comparing Ti and Zi with and without fixed ceramic material, all of the materials caused visible ΔE when the gingival thickness was equal to 1.5 mm. At 2 mm, only Ti caused a visible ΔE. Finally, when the gingival thickness was ≥ 3 mm, no material caused a visible ΔE. This study clearly establishes an association between gingival thickness and the indication of the material of choice, in relation to the gingival thickness.
A prospective randomized study by this same group of researchers,
9 comparing aluminium oxide abutments with titanium ones, showed an ΔE that was significantly lower for the aluminium oxide. However, the group with an aluminium oxide abutment had a mean gingival thickness of 3.4 ± 1.4 mm, against a thickness of 2.9 ± 0.9 mm in the titanium abutment group. This mean difference of 0.5 mm might, at least in part, explain the difference between those two thicknesses.
In a prospective study, Andersson et al.
16 divided patients in two groups (≤ 2 mm and > 2 mm of thickness) and concluded that there was no association between gingival thickness and induced color change and that titanium, zirconia, and gold resulted in a visible ΔE even though the values were significantly less significant for gold and zirconia, compared to titanium. Nevertheless, it should be clarified that the patients in the sample had a quite thin gingiva as there was no patient with a gingival thickness of ≥ 3 mm.
In the study by Zembic et al.,
25 both zirconia and titanium caused visible ΔE, similar in value. In this study, gingival thickness was approximately 1.9 ± 0.8 in the zirconia arm and 1.7 ± 0.4 mm in the titanium arm. van Brakel et al.
21 came to the conclusion that no visible ΔE was acquired from a thickness equal or greater than 2 ± 0.1 mm, either in titanium or zirconia. Cosgarea et al.
13 stated that when mean gingival thickness ranged from 1.02 ± 0.36 mm to 2.27 ± 0.34 mm, titanium and zirconia abutments both resulted in significant final visible ΔEs, with no association with gingival thickness having been noted. One must also note the small mean gingival thickness of this particular sample and the absence of a graft case.
Evidently, there is lack of standardization among the studies and the different results do not allow the establishment of an exact limit of gingival thickness, beyond which no type of material results in visible discolouration. The only study clearly establishing an association between gingival thickness and the material used, an association characterized by ΔE inferior to the visible threshold, was an
in vitro study.
13 However, all
in vivo studies seemed to converge towards the fact that, as long as the mean gingival thickness of the sample was ≤ 2 mm, the implant abutment caused a visible change to the gingival color, no matter what the material was. Zirconia and aluminium oxide caused a color variation that was less pronounced but still remained visible. In our results, only Standard shade zirconia caused a non visible color change in thin gingiva.
For the majority of clinical studies, abutments made of zirconia (thought to be more aesthetic) seem to cause gingival discolouration to a lesser degree than titanium abutments. This discolouration remains visible in certain cases. Other strategies are being studied, aiming to improve the aesthetics of implant-supported restorations of the anterior sector.
In a retrospective clinical study, Happe et al.
26 proposed individualised zirconia abutments, modified with a 2 mm ceramic neck, of clear orange fluorescent color to 12 patients needing an implant in the anterior sector,. The results, obtained with spectrophotometric analysis, were promising; the color variation induced in 5 of the 12 patients was below the threshold and was visible to the naked eye. However, it must be made clear that this particular study excluded patients with a gingival thickness of ≤ 2 mm. The gingival biotype was quite thick, a factor that could partially explain the results. Ishikawa-Nagai et al.
27 equally obtained the results below the threshold distinguishable to the naked eye, proposing the implants with a neck of light pink or light orange color. The idea of using the most natural colors in order to mimic the natural gingiva seems equally interesting.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download