Abstract
PURPOSE
This study evaluated the effect of chemical surface treatment using methyl formate-methyl acetate (MF-MA) solution on the tensile bond strength between acrylic denture teeth and auto-polymerized acrylic resin.
MATERIALS AND METHODS
Seventy maxillary central incisor acrylic denture teeth for each of three different brands (Yamahachi New Ace; Major Dent; Cosmo HXL) were embedded with incisal edge downwards in auto-polymerized resin in polyethylene pipes and ground with silicone carbide paper on their ridge lap surfaces. The teeth of each brand were divided into seven groups (n=10): no surface treatment (control group), MF-MA solution at a ratio of 25:75 (v/v) for 15 seconds, 30 seconds, 60 seconds, 120 seconds, 180 seconds, and MMA for 180 seconds. Auto-polymerized acrylic resin (Unifast Trad) was applied to the ground surface and polymerized in a pressure cooker. A tensile strength test was performed with a universal testing machine. Statistical analysis of the results was performed using two-way analysis of variance (ANOVA) and post-hoc Dunnett T3 test (α=.05).
RESULTS
The surface treatment groups had significantly higher mean tensile bond strengths compared with the control group (P<.05) when compared within the same brand. Among the surface treatment groups of each brand, there were no significantly different tensile bond strengths between the MF-MA groups and the MMA 180 second group (P>.05), except for the Yamahachi New Ace MF-MA 180-second group (P<.05).
The most common type of acrylic denture failure is debonding or fracture of the denture teeth, accounting for approximately 33% of failures.1 This failure usually occurs in the anterior region of the denture. Dentists and patients both prefer chair-side repair using auto-polymerized acrylic resin, because it is a straightforward procedure and takes less chair time than repair in the laboratory. A likely explanation for teeth debonding is weak bond due to contamination of the tooth surfaces with substances such as wax during laboratory processing. Another cause is a chemical difference between the tooth and denture base because of different processing methods.2 Weakness of the repaired interface leads to recurrent debonding. Therefore, the surface treatment method, mechanical or chemical, is important for denture repair. The placement of a diatoric recess (mechanical) and the use of a bonding agent (chemical) on denture teeth resulted in higher bond strengths compared with no treatment.34
Methyl methacrylate (MMA) is the most frequently used chemical surface treatment agent. MMA is effective as an adhesion promoter because of its chemical similarities to denture base. However, surface treatment with MMA requires 3 minutes to effectively prime the surface for ultimately reducing adhesive failure,5 thus requiring an excessive time. Other solutions have been used as chemical surface treatments (e.g., chloroform, methylene chloride (dichloromethane), and 4-methacryloxyethyl trimellitate anhydride (4-META)). Although chloroform and methylene chloride have been used, they have been identified as being carcinogenic.6 Researchers6 found that two solutions, methyl formate and methyl acetate, were non-toxic and resulted in similar bond strengths to poly(methyl methacrylate) to those obtained with methylene chloride. Another study found that methyl formate, methyl acetate, and their mixture significantly enhanced the flexural strength of heat-cured acrylic denture base resin that had been repaired with self-cured acrylic resin. The scanning electron micrographs in this study demonstrated that the application of these solutions to heat-cured acrylic resin resulted in a 3D honeycomb appearance, whereas specimens treated with methyl methacrylate developed shallow pits.7 In addition, other studies have been conducted for investigating the surface treatment of acrylic denture base and reline resin with methyl formate-methyl acetate (MF-MA). These studies have shown that MF-MA significantly enhanced the bond strength between acrylic denture base and reline resin.89
A study of MF-MA application on acrylic denture teeth revealed that a 15-second application before packing the teeth with heat-cured acrylic denture base resulted in significantly higher micro-tensile bond strength between the teeth and the denture base, compared with the no surface treatment group. Moreover, there was no significant difference between the MF-MA and MMA groups using a 15-second application on micro-tensile bond strength.10 Therefore, MF-MA may be an acceptable alternative for MMA because it is less toxic.
The acrylic denture tooth structure can also influence the bond strength to the denture.311 The conventional tooth has low wear resistance. Therefore, increased wear resistance is useful,12 and cross-linking the polymer matrix can increase wear resistance.13 Because of this complex structure, the acrylic has less polymer penetrability than into conventional denture teeth, resulting in lower bond strength. In contrast, another study stated that there was no significant difference in bond strength between conventional and cross-linked denture teeth to auto-polymerized acrylic resin.14 Thus, crosslinking is not a major factor in reducing the strength of the joint between teeth and denture base.15
There are various methods for determining the bond strength between denture teeth and denture base, such as the American Dental Association Specification number (ADA 15),16 International Organization for Standardization for synthetic resin teeth (ISO 3336),17 or the finite element stress analysis technique.16 However, these methods have been criticized concerning their accuracy in determining bond strength. Moreover, the lack of uniformity in the tooth-denture base testing methods does not allow bond strength to be investigated in a standardized manner and/or the results to be directly compared.2
Past studies using MF-MA solutions have indicated that it is a non-toxic surface treatment agent and improves the bond strength of acrylic resin materials. However, there are no studies of the bond strength when using MF-MA for the repair of denture teeth with auto-polymerized acrylic resin. The objective of this study was to evaluate the effect of surface treatment with MF-MA solution, using various application times and comparing to that of MMA, on the tensile bond strength of different acrylic denture teeth repaired with auto-polymerized acrylic resin.
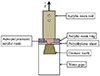
Three brands of denture teeth were used: Yamahachi New Ace (YA), Major Dent (MD), and Cosmo HXL (CM), (Table 1). Seventy maxillary central incisor acrylic denture teeth of each brand were embedded with incisal surface down in auto-polymerized poly(methyl methacrylate) (PMMA) and were packed in 20-mm diameter polyethylene pipes. Their ridge-lap surfaces were polished with 500- and 1200-grit silicon carbide paper in a polishing machine (Nano2000, PACE Technologies, St. Tucson, AZ, USA). The specimens of each brand were divided into groups of 10 specimens each. Chemical solutions were applied to the groups as follows (Table 2): no treatment; methyl formatemethyl acetate (MF-MA) solution at a ratio of 25:75 (by volume) (CU Acrylic Bond, Faculty of Dentistry, Chulalongkorn University, Bangkok, Thailand) for 15 seconds, 30 seconds, 60 seconds, 120 seconds, 180 seconds, and MMA for 180 seconds. A polyethylene sheet with a 3-mm diameter hole was placed over the treated surface and a 10-mm diameter acrylic resin ring was placed centrally over the hole in the polyethylene sheet. Auto-polymerized acrylic resin (Unifast Trad, GC Dental Products Co., Aichi, Japan) was loaded into the ring and compressed with a 1-kg weight. The specimen was placed in a pressure cooker at 2 MPa at 60℃. After the acrylic resin had set, an acrylic resin rod was attached on the top with cyanoacrylate glue (Super Glue, Alteco Chemical PTE Ltd., Japan) to connect the specimen to the tensile testing machine (Fig. 1). The polymerized specimens were stored in deionized water at 37℃ for 48 ± 2 hours and tested using a universal testing machine (Shimadzu, EZ-S, Bara Scientific Co., Ltd., Phra Nakhon Si, Ayutthaya, Thailand) with a 500 N load cell at a crosshead speed of 10 mm/min. The tensile bond strength (in MPa) was calculated by dividing the failure force by the bond surface area.
The data were statistically analyzed using SPSS for Windows 22 (IBM Corporation, New York, NY, USA). The mean and standard deviation (SD) for the tensile bond strength of each group was calculated and statistically analyzed using two-way analysis of variance (ANOVA) and the post hoc Dunnett T3 test at the 95% confidence level.
The results (Table 2) demonstrated that the tensile bond strengths of the negative control groups (YAC; MDC; CMC) were not significantly different from each other (P > .05). For each brand, the surface treatment groups had significantly higher tensile bond strengths compared with the negative control group (P < .05). The tensile bond strengths of the MMA 180-second group of each brand were not significantly different from the MF-MA 15-second, 30-second, 60-second, 120-second, and 180-second groups (P > .05). However, the YA MF-MA 180-second group had a significantly higher tensile bond strength compared with the MMA 180-second group (P < .05).
The bond strength of acrylic denture teeth to auto-polymerized acrylic resin can be improved by chemical surface treatment. This improvement begins when the solvents in the surface treatment agent contact the denture teeth, dissolving their surfaces, and causing swelling of the surface layers. Subsequently, the monomer of the auto-polymerized acrylic resin material diffuses and penetrates into the interpenetration polymer network (IPN) matrix of the denture teeth during polymerization, which is known as the swelling phenomenon.17 This mechanism is affected by application time, polymerization temperature, type of solvent, denture teeth structure, and glass transitional temperature of the denture teeth.18
When used on acrylic denture teeth as a surface treatment, a methyl formate-methyl acetate solution acts by swelling and dissolving their surfaces, and then evaporating. In addition, there are no carbon–carbon double bonds (C=C) in methyl formate or methyl acetate molecules to polymerize with the monomer in the auto-polymerized acrylic material. Thus, it would not obstruct the interlocking of the auto-polymerized resin polymer chains and the denture teeth, and the tensile bond strength would be increased.
Polymer dissolving and swelling occurs when the polymer's and solvent's solubility parameters and polarities are close to each other. The solubility parameter of PMMA (acrylic denture teeth) is 18.3 MPa1/2, whereas those of MMA, MF, and MA are 18.0, 20.9, and 19.6 MPa1/2, respectively.19 In addition, MMA, MF, and MA molecules have the methyl ester group, which increases their ability to soften PMMA.6 The MF, MA, and MF-MA mixture increased the bond strength of repaired acrylic denture base and acrylic denture base relined with rebasing material.78 Thus, it is hypothesized that MF-MA mixture would result in higher tensile bond strength between acrylic denture teeth and auto-polymerized acrylic resin compared with using MMA liquid.
Although the results indicated that surface treatments with MF-MA and MMA improved the bond strength compared to no treatment, MF-MA was superior to MMA for the three brands of denture teeth for chairside repairs due to MF-MA's reduced application time and lack of tissue irritation. The bond strengths when using MMA for 180 s for all brands was not significantly different compared with the MF-MA 15 second, 30 second, 60 second, 120 second, and 180 second groups (P > .05), and MF-MA treatment resulted in bond strengths similar to MMA treatment. The shortest application time of MF-MA treatment that had a higher bond strength compared with the control was 15 seconds, thus is the best time for chairside use for both conventional and cross-linked acrylic denture teeth.
According to denture teeth types, the results showed that the bond strengths between cross-linked (Cosmo HXL) and conventional type (Yamahachi New Ace; Major Dent) were not significantly different (P > .05), the result which agrees with a previous study.15 The reason may be the type of acrylic denture base because the bond repaired with heat-cured acrylic resin was greater than that with auto-polymerized resin.20 The stronger bond is the result of processing temperature, time, and pressure. These factors promote monomer penetration, especially in conventional acrylic denture teeth. The polymer chain network size in conventional acrylic denture teeth is larger compared with that in the cross-linked type.21 Therefore, there is more space between the polymer chains in conventional acrylic denture teeth compared with the cross-linked type (crosslink density). Thus, monomer from the auto-polymerized acrylic resin can diffuse more into the conventional acrylic denture teeth, resulting in increased polymerization. Therefore, denture tooth type influences the bond strength for heat-cured acrylic resin, but not for auto-polymerized acrylic. Another reason may be that the cross-linked structure of Cosmo HXL, an interpenetrating polymer network (IPN) consisting of only 10% highly crosslinked PMMA resin,22 is not complex enough to result in a different bond strength. If the denture tooth has a true IPN structure, the tensile bond strength between the tooth and auto-polymerized resin will be reduced because IPN denture tooth swells less after surface treatment with chemical agents (MF-MA, MMA).
A limitation of this study was that only conventional and cross-linked denture teeth were investigated. The IPN denture teeth have an advantage of high wear resistance.13 This type of tooth is appropriate for dentures that occlude natural teeth. However, its bond strength to the denture base is low. The IPN consists of two or more polymer networks, resulting in a more complex structure compared with the cross-linked type. Therefore, this type of tooth should also be studied because it will generate useful data for determining the most suitable surface treatment method in each case.
Within the limitations of the present study, CU Acrylic Bond (MF-MA solution) and MMA increased the bond strength of either conventional or crosslinked acrylic denture teeth to auto-polymerized acrylic resin compared to no treatment. The application of MF-MA for 15 second can be an alternative chemical surface treatment for repairing a denture base and rebonding both conventional and cross-linked acrylic denture teeth with auto-polymerized acrylic resin.
Figures and Tables
Table 1
Materials used in this study

Table 2
Multiple comparisons of the means (± standard deviations) of the tensile bond strengths (in MPa), according to the group

References
1. Darbar UR, Huggett R, Harrison A. Denture fracture-a survey. Br Dent J. 1994; 176:342–345.
2. Cunningham JL. Bond strength of denture teeth to acrylic bases. J Dent. 1993; 21:274–280.
3. Takahashi Y, Chai J, Takahashi T, Habu T. Bond strength of denture teeth to denture base resins. Int J Prosthodont. 2000; 13:59–65.
4. Meng GK, Chung KH, Fletcher-Stark ML, Zhang H. Effect of surface treatments and cyclic loading on the bond strength of acrylic resin denture teeth with autopolymerized repair acrylic resin. J Prosthet Dent. 2010; 103:245–252.
5. Vallittu PK, Lassila VP, Lappalainen R. Wetting the repair surface with methyl methacrylate affects the transverse strength of repaired heat-polymerized resin. J Prosthet Dent. 1994; 72:639–643.
6. Asmussen E, Peutzfeldt A. Substitutes for methylene chloride as dental softening agent. Eur J Oral Sci. 2000; 108:335–340.
7. Thunyakitpisal N, Thunyakitpisal P, Wiwatwarapan C. The effect of chemical surface treatments on the flexural strength of repaired acrylic denture base resin. J Prosthodont. 2011; 20:195–199.
8. Osathananda R, Wiwatwarrapan C. Surface treatment with methyl formate-methyl acetate increased the shear bond strength between reline resins and denture base resin. Gerodontology. 2016; 33:147–154.
9. Kungkapilas K, Santawisuk W. Effect of chemical surface treatment on bond strength of a heat-cured acrylic resin denture base and a reline resin. Ciang Mai Dent J. 2014; 35:51–61.
10. Penpattanakul S, Wiwatwarrapan C. Effect of chemical surface treatment on microtensile bond strength between acrylic denture teeth and heat-cured acrylic denture base. In : Proceeding The 1st Asean Plus Three Graduate Research Congress; 1-2 Mar. 2012; Chaingmai, Thailand. ST232-7.
11. Kawara M, Carter JM, Ogle RE, Johnson RR. Bonding of plastic teeth to denture base resins. J Prosthet Dent. 1991; 66:566–571.
12. Suwannaroop P, Chaijareenont P, Koottathape N, Takahashi H, Arksornnukit M. In vitro wear resistance, hardness and elastic modulus of artificial denture teeth. Dent Mater J. 2011; 30:461–468.
13. Coffey JP, Goodkind RJ, DeLong R, Douglas WH. In vitro study of the wear characteristics of natural and artificial teeth. J Prosthet Dent. 1985; 54:273–280.
14. Clancy JM, Boyer DB. Comparative bond strengths of light-cured, heat-cured, and autopolymerizing denture resins to denture teeth. J Prosthet Dent. 1989; 61:457–462.
15. Anderson JN. The strength of the joint between plain and copolymer acrylic teeth and denture base resins. Br Dent J. 1958; 104:317–312.
16. Darbar UR, Huggett R, Harrison A, Williams K. The tooth-denture base bond: stress analysis using the finite element method. Eur J Prosthodont Restor Dent. 1993; 1:117–120.
17. Vallittu PK, Ruyter IE, Nat R. The swelling phenomenon of acrylic resin polymer teeth at the interface with denture base polymers. J Prosthet Dent. 1997; 78:194–199.
18. Hopfenberg HB, Stannett V. The diffusion and sorption of gases and vapours in glassy polymers. In : Haward RD, editor. The Physics of glassy polymers. London: Applied Science Publishers;1973. p. 504–547.
19. Grulke EA. Solubility parameter values. In : Brandrup J, Immergut EH, Grulke EA, editors. Polymer Handbook. 4th ed. New York: Wiley;1999. p. 696–697. p. 708
20. Civjan S, Huget EF, de Simon LB. Modifications of the fluid resin technique. J Am Dent Assoc. 1972; 85:109–112.
21. Suzuki S, Sakoh M, Shiba A. Adhesive bonding of denture base resins to plastic denture teeth. J Biomed Mater Res. 1990; 24:1091–1103.
22. Hao Z, Yin H, Wang L, Meng Y. Wear behavior of seven artificial resin teeth assessed with three-dimensional measurements. J Prosthet Dent. 2014; 112:1507–1512.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download