Abstract
PURPOSE
The purpose of this study was to analyze the antimicrobial, antioxidant activity and cytotoxicity of Dendropanax morbifera Léveille extract for assessing whether Dendropanax morbifera Léveille can be used for the development of natural mouthwash and denture cleaning solution.
MATERIALS AND METHODS
The extract was obtained from branches of Dendropanax morbifera Léveille. The solvent fractions were acquired by fractionating Dendropanax morbifera Léveille extract using n-hexane, ethyl acetate, chloroform and butanol solvent. Paper disc test was used to evaluate the antimicrobial and antifungal activity of Dendropanax morbifera Léveille extract and solvent fractions against Streptococcus mutans and Candida albicans. The analysis of antioxidant activity was carried out through DPPH radical scavenging assay. The cytotoxicity of Dendropanax morbifera Léveille extract was analyzed through MTT assay using normal human oral keratinocytes.
RESULTS
Dendropanax morbifera Léveille extract showed antimicrobial activity against Streptococcus mutans and especially Candida albicans. The solvent fractions of Dendropanax morbifera Léveille showed strong antimicrobial activity against Streptococcus mutans and Candida albicans in n-hexane and butanol solvent fraction, respectively. Dendropanax morbifera Léveille extract also showed outstanding antioxidant activity. Butanol, ethyl acetate, and chloroform solvent fraction of Dendropanax morbifera Léveille tended to have increased antioxidant activity as the concentration increased. Dendropanax morbifera Léveille extract showed high cell survival rate in cytotoxicity test.
Dentate patients must exercise strict hygiene control through the use of oral hygiene products (toothbrush, mouthwash, etc.) in order to maintain healthy teeth and gums. Similarly, patients using complete or partial dentures must also exercise hygiene control, in order to maintain the health of the remaining teeth and gums and for continued, problem-free use of the dentures for a long period of time. However, the dentist-initiated recall checks of patients using dentures have revealed many cases of poor hygiene control of the mouth and dentures; this has also been observed in studies on bacterial growth in denture patients suffering from stomatitis, or on the surface of denture. Glass et al.1 reported the presence of over 900 types of aerobic and anaerobic bacteria in the biofilm of dentures. Another study also reported the frequent occurrence of stomatitis in patients using dentures; Candida albicans (C. albicans), the main causative bacteria of stomatitis, was found in approximately 86% of these patients.2 Bacteria, such as Streptococcus mutans (S. mutans) and Streptococcus salivaris (S. salivaris), present on the surface of the acrylic resin, which is the major component of dentures, or in the oral epithelium, are responsible for the development of dental caries in the remaining teeth; in addition, these bacteria acidify the environment within the mouth, fostering conditions favorable for the propagation and adhesion of C. albicans.3 Therefore, S. mutans and S. salivaris are believed to induce active proliferation of C. albicans in the biofilm of dentures, or within the mouth.45 These studies emphasized the importance of oral hygiene and cleaning of dentures; the studies also focused the efficacy of physical cleaning such as tooth brushing.6 However, physical cleaning could be an issue for elderly patients, who lack awareness regarding oral and denture cleaning, or who face physical limitations. Various types of mouthwash and denture cleaning solutions have been developed and are currently available for such patients.
Most of commercially available mouthwash and denture cleaning solutions are prepared with chemical components. The cytotoxicity of the chemical components of mouthwash solutions, in particular, poses a major problem, because of their direct contact with oral tissues. Therefore, the current focus is on developing cleaning agents that contain easily available natural products with superior biocompatibilityand a low cost of development. It has been predicted that mouthwash and denture cleaning solutions prepared from natural products will be developed and utilized gradually in clinical settings, based on the knowledge that over 75% of the medicines and drugs developed against bacterial infections are directly extracted from natural products or their derivatives.7
Recent studies have developed mouthwash and denture cleaning solutions from the active ingredients of sea weeds (sweet laver; Eckloma cava), medicinal herbs (Lophatheri herba; Lophatherum gracile Brongn), or tall trees (Japanese cypress; Chamaecyparis obtusa); these products are currently available in the market. Some of these natural products are proven to have antibiotic and antioxidant effects, and can be used as mouthwash and denture cleaning solutions; however, they cannot be ingested. As a small quantity of the mouthwash or denture cleaning solution might inadvertently be left behind in the mouth or swallowed, it would be safer to use potable natural products in the formulation of these solutions. Therefore, Korean Dendropanax (Dendropanax morbifera Léveille), a potable natural product with multiple healing effects, has been used for various medicinal purposes and can be applied to the formulation of mouthwash and denture cleaning solutions.
Dendropanax morbifera Léveille is a tree that grows mainly in the Jeju Island, and some portions of the Korean coastline along the southwestern sea. Traditionally, it has been used to prepare tea or medicine for liver disease and immunological enhancement. Dendropanax morbifera Léveille extracts have been reported to exert various effects, including immune activation against cancer cells, antioxidant effects,89 diabetes treatment and prevention,10 and insecticidal effects.11 In addition, the anticomplementary12 and antibiotic effects, and the medicinal effects of Dendropanax morbifera Léveille on liver diseases have also been studied. In particular, Dendropanax morbifera Léveille has been reported to express a superior antiproliferative activity against various microbes. Lee et al.,13 in a related study, reported the antibiotic activity of Dendropanax morbifera Léveille against Staphylococcus aureus. Another study14 performed by the Korea Forest Research Institute, which investigated the biological activities of trees listed Dendropanax morbifera Léveille among the trees containing components with anti-dental caries activity. However, almost no study in dentistry has been conducted using Dendropanax morbifera Léveille extracts. This study evaluated the antibiotic and antifungal effect, antioxidant capacity, and cell toxicity of the bough extracts and solvent fractions of Dendropanax morbifera Léveille. We believe that the results of this study could be used as the starting point to evaluate the feasibility of development of constituent mouthwash and denture cleaning solutions in the future.
The boughs of Dendropanax morbifera Léveille were collected from a natural habitat in Haenam-gun, Jeollanam-do, during February 2013. These were dried at room temperature, and subjected to the extraction process. The collected Dendropanax morbifera Léveille boughs were cut into 1.0 cm length sections. The sectioned Dendropanax morbifera Léveille boughs (500 g) were collected in a Pyrex container (5 L) for extraction; the extraction was performed in triplicate at 50℃ for 5 hours with 94% (v/v) ethanol solvent (5 L), using a JAC ultrasonic 4020 (KODO, Daejeon, South Korea). The extracts were filtered thrice through a Whatman filter paper No. 5 (Whatman Bioscience, Cambridge, UK). The filtrates at the lower layer were separated into a Florence flask for decompression; the filtrates were concentration by decompressing with a vacuum evaporator, in order to evaporate ethanol.
The Dendropanax morbifera Léveille extracts were subjected to solvent fractionations using 4 different solvents under a chemical hood in order to investigate the extracts in detail. The 16 g of extracts were completely resuspended in 300 mL distilled water; 300 mL n-hexane was added to the resuspension, and the resultant mixture hand shaken for 10 minutes. This mixture was left on the table until the complete separation of the water and n-hexane layers. The upper n-hexane layer was transferred to a beaker without disturbing the water layer. The entire procedure was repeated 3 times; the obtained n-hexane layer was concentrated using a vacuum concentrator, and dried in a 50℃ dry oven for over 30 hours in order to obtain a pure n-hexane solvent layer. Solvent fractionations were also performed with chloroform, ethyl acetate, and butanol using the above-mentioned procedure. Each solvent fraction was stored in a refrigerator until further analysis.
This experiment utilized the oral microbes C. albicans (ATCC 90028) and S. mutans (KCTC 3065) obtained from the clinical research laboratory of the Chosun University Dental College. The initial cultures of C. albicans and S. mutans were prepared in Luria-Bertani (LB) broth and brain heart infusion agar (BHI), respectively, at 37℃ for 24 - 48 hours. The initial cultures were sub-cultured thrice, and subsequently used in the analysis of antibiotic activity. The bacterial concentration was determined by measuring the absorbance at 650 nm using a spectrophotometer (ELISA reader; Bio-Tek Instruments Inc., Winooski, VT, USA); the bacterial concentration to be inoculated to the analytical agar plates was measured by adjusting the absorbance to 0.4 - 0.5/1 mL.
The antiproliferative activity of antimicrobial compounds was determined by the paper disc method,15 which could be used to verify the antibiotic and antifungal effects. The concentration of the extracts and solvent fractions were adjusted to 20, 40, 80, and 100 µg/mL with 100% diluted dimethyl sulfoxide (DMSO; Sigma-Aldrich Co, St. Louis, MO, USA). Each of these solutions was absorbed onto an 8 mm paper disc (Advantec Co, Saijyo, Japan). The S. mutans and C. albicans strains were cultured at 37℃ for 24 hours with these discs. The diameter of the clear zone radiating from the disc was measured in order to identify the degree of antiproliferative activity of the extracts.
The antioxidant capacity of the extracts and solvent fractions was analyzed by measuring their free radical scavenging activity, using the DPPH radical scavenging assay.16 The samples were prepared at concentrations of 31.3, 62.5, 125, 250, and 500 µg/mL. Vitamin C (Vit. C) and butylated hydroxytoluene (BHT) were used in the positive control group. The samples were mixed with 500 µM DPPH solution (1:1 ratio), and incubated in the dark at room temperature for over 30 minutes. The free radical scavenging activity was determined by measuring the absorbance at 517 nm using a spectrophotometer. The results of the DPPH radical scavenging activity were analyzed by one-way analysis of variance (ANOVA) using the SPSS 21.0 software (IBM, Armonk, NY, USA). The statistical significance was determined by Tukey's multiple comparison test, at a significance level of 5% (P < .05).
Normal human oral keratinocyte obtained from Science Cell Research Laboratories (Carlsbad, CA, USA), and cultured according to the manufacturer-recommended protocol, were used in the cytotoxicity experiments.
The cells were sub-cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin (P/S; Gibco) every 24 - 36 hours. Aliquots of the sub-cultures were added to 96-well culture plates at a concentration of 1 × 104 cells/well; these plates were incubated at 37℃ for 24 hours in a 5% CO2 incubator.
The cytotoxicity of all samples was evaluated using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The HNOK cells were treated with 200 µL Dendropanax morbifera Léveille extracts at varying concentrations (0, 2.5, 5, 10, 25, and 40 µg/mL); the reaction mixtureswere incubated at 37℃ for 20 hours in a 5% CO2 incubator. The MTT solution (20 µL; Sigma-Aldrich) was added to each mixture, and the reaction mixtures incubated at 37℃ for 4 hours in a 5% CO2 incubator. The culture medium containing the MTT solution was removed after incubation. Normal human oral keratinocyte was dissolved in 200 µL DMSO (Sigma-Aldrich), and the absorbance of this mixture measured at 540 nm using a spectrophotometer (ELISA reader; Bio-Tek Instruments Inc.). This experiment was performed in triplicate.
The antiproliferative activity of Dendropanax morbifera Léveille extracts on S. mutans are presented in Fig. 1A and Table 1. The diameter of S. mutans clear zone was unclear at an extract concentration of 20 µg/mL; however, Dendropanax morbifera Léveille extracts at concentrations of 40, 80, and 100 µg/mL resulted in clear zones of 2.5, 3.0, and 3.0 - 3.5 mm diameter, respectively.
The antiproliferative activity of Dendropanax morbifera Léveille extracts at 20 µg/mL against C. albicans was indicated by a clear zone with a diameter of 2.0 mm; a clear zone of 2.0 - 2.5 mm was observed at extract concentrations of 40 and 80 µg/mL, and a 3.0 mm clear zone was observed when the concentration was 100 µg/mL (Fig. 1B, Table 1).
While the lowest concentration (20 µg/mL) of Dendropanax morbifera Léveille extracts did not show a clear antibiotic effect against S. mutans, it showed antifungal activity against C. albicans at the same concentration. This suggested the higher sensitivity of the extracts against C. albicans, although the extracts showed similar antibiotic effects against S. mutans and C. albicans at concentrations higher than 40 µg/mL.
Four types of organic solvent fractions were produced from the ethanol extracts of Dendropanax morbifera Léveille The antiproliferative activity of each fraction against S. mutans and C. albicans was investigated. The antiproliferative activities of the Dendropanax morbifera Léveille solvent fractions against S. mutans are presented in Fig. 2 and Table 2. The n-hexane fraction showed a much higher antibiotic activity compared to other fractions; the antibiotic activity against S. mutans increased in proportion to the concentration of the extracts used to prepare the solvent fractions. The n-hexane solvent fraction discs prepared from extract concentrations of 20, 40, 80, and 100 µg/mL resulted in clear zone diameters of 1 - 1.5, 1.5 - 2.3, 2 - 2.5, and 2.3 - 3 mm, respectively. Although the ethyl acetate fraction showed a lower antibiotic activity than the n-hexane fraction, it exerted an antibiotic effect (< 0.5 mm) even at extract concentrations of 20 and 40 µg/mL unlike the chloroform and the butanol fractions. The diameter of the clear zone was 1 mm at extract concentrations of 80 and 100 µg/mL. The chloroform fraction showed no antibiotic activity at lower extract concentrations (20 and 40 µg/mL); however, its antibiotic activity increased considerably at 100 µg/mL (1 - 1.5 mm). The butanol fraction also showed no clear zone at 20 and 40 µg/mL; the discs prepared from solvent fractions of 80 and 100 µg/mL extracts effected weak antibiotic activities (0 - 0.3 and 0 - 0.5 mm diameter clear zones, respectively).
Only the butanol solvent fraction among the four types showed antifungal antiproliferative activity at an extract concentration of 20 µg/mL against C. albicans (Fig. 3, Table 3). At concentrations of 40, 80, and 100 µg/mL, the butanol solvent fractions resulted in clear zones that were 1.0, 1.0 - 1.5, and 1.5 mm wider, respectively, compared to the other fractions. The n-hexane fraction showed the obvious antifungal activity after the butanol fraction, with 0.2 - 1.2 mm clear zones at concentrations higher than 40 µg/mL. The chloroform and ethyl acetate fractions resulted in consistent clear zones 0.5 mm in diameter at concentrations higher than 40 and 80 µg/mL, respectively.
DPPH radical scavenging activities of the Dendropanax morbifera Léveille extracts are listed in Table 4. The Dendropanax morbifera Léveille extracts displayed similar DPPH radical scavenging activity patterns to BHT, a synthetic antioxidant, within a range of 31.3 - 62.5 µg/mL. However, at the highest concentration (500 µg/ml), it showed a much higher DPPH radical scavenging activity (82.92 ± 0.49%) compared to BHT (56.71 ± 6.34%). A comparison of these results with that shown by vitamin C, the positive control group, revealed similar antioxidant capacities of the Dendropanax morbifera Léveille extracts (82.92 ± 0.49%) and vitamin C (90.11 ± 0.13%) at the same concentration of 500 µg/mL; the antioxidant activity of vitamin C was greater (by approximately 40%) than that of BHT (56.71 ± 6.34%) at the same concentration (500 µg/mL).
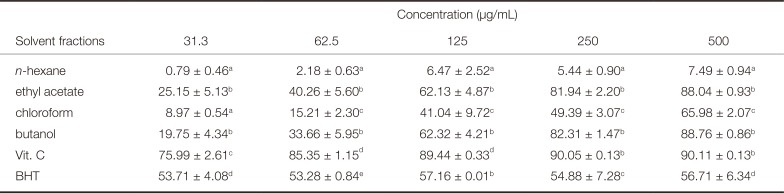
The DPPH radical scavenging activities of the Dendropanax morbifera Léveille solvent fractions are listed in Table 5. Among the four Dendropanax morbifera Léveille solvent fractions, the n-hexane fraction showed a very weak DPPH radical scavenging activity (< 10%) at the highest concentration (500 µg/mL). On the other hand, the DPPH radical scavenging activities of the fractions prepared using ethyl acetate, chloroform, and butanol increased proportionally with the increase in concentration. The chloroform fraction showed a > 60% scavenging activity at 500 µg/mL. The ethyl acetate and the butanol fractions showed similar antioxidant capacities; both fractions showed DPPH radical scavenging activities > 60%, with a maximum of > 85% at a concentration range of 125 - 500 µg/mL. Higher antioxidant capacities were observed at a concentration of 500 µg/mL than that shown by BHT, a synthetic antioxidant.
Dendropanax morbifera Léveille extracts retained a high cell viability (60 - 100%); the effective concentration range without affecting the survival rate was 2.5 - 10 µg/mL, than that of the control group. The remaining concentration groups (25 - 40 µg/mL) displayed a survival rate of approximately 70% (Fig. 4).
With the increase in patient awareness on oral hygiene control, study groups have attempted to develop various oral hygiene products. In particular, special hygiene control or cleaning products have been recommended for patients who use removable dental prostheses, as the control of denture hygiene, as well as that of the remaining teeth and gums. Most of the currently available mouthwash solutions are prepared from synthetic chemical components, and contain chlorhexidine as the antimicrobial component. Chlorhexidine is a cathionic broad spectrum antimicrobial compound belonging to bis-biguanide family, which is known to inhibit the adhesion of microbes on the oral mucosa or the surface of dentures.17 This component exerts cytostatic activity by increasing the cell membrane permeability at low concentrations and inducing cytoplasmic deposition at high concentrations.18 Therefore, it has been extensively used in denture cleaning and mouthwash solutions. However, the major drawback of chlorhexidine is that long term use results in discoloration of the teeth and dentures. In addition, patients with oral disease or elderly patients with impaired movement of the tongue or oral muscles may ingest these solutions during usage. Therefore, it needs to be prepared and handled carefully. Consequently, in order to reduce the risks of tooth discoloration and ingestion, attempts have been made to develop mouthwash and denture cleaning solutions using safer natural extracts with effects that are comparable to chemical cleaning agents. The Korean Food Standards Codex has recorded the leaves, stems, and roots of Dendropanax morbifera Léveille to be edible. In addition, these have been traditionally used as a tea, or as materials for decoction19 because of their functional bioactive effects against cancer, in detoxification, and diabetes treatment. Therefore, it has been considered to be safe for usage in the mouth, compared to extracts from saplings that are impossible to drink, like the Japanese cypress.
Although a limited number of studies have investigated the use of Dendropanax morbifera Léveille extracts, many of these studies have reported on the uses of leaf, bough, and root extracts. However, the roots of Dendropanax morbifera Léveille have limited usage because of the lifespan of the tree, while the properties and harvest of the leaves vary with the season. Therefore, this study investigated the boughs of Dendropanax morbifera Léveille, with an expected high bioavailability. As solvent fractionation is used to analyze the functionality of the extracts in detail, this study also utilized bough solvent fractions as analytical samples. The solvent fractions were obtained using 4 different organic solvents, in addition to ethanol, which was used for extraction.
In order to use natural extracts as mouthwash and denture cleaning solutions, their antibacterial and antifungal effects against major groups of bacteria that adhere to the mouth and dentures must be verified. S. mutans is a representative causative organism20 for dental caries. The acidic environments resulting from the formation of organic acids during glucose metabolism promote the adhesion of C. albicans to the oral mucosa and dentures.3 In addition, most polymicrobial biofilms that are formed on removable dental prostheses are composed of Staphylococcus and Candida sp.3 In addition, some studies have observed a rapid increase in α-hemolytic Streptococcus and C. albicans in patients with denture stomatitis.21 These support the theory that S. mutans and C. albicans are the major stomatitis causative microbes in patients using dentures. Therefore, this study analyzed the antibacterial and antifungal activities of Dendropanax morbifera Léveille extracts and solvent fractions against S. mutans and C. albicans.
A report prepared by the Korean Ministry of Science and Technology22 revealed that the Dendropanax morbifera Léveille leaf extracts showed a strong antibacterial effect against gram positive bacteria, and a strong antifungal effect against C. albicans. Another study11 reported that Dendropanax morbifera Léveille extracts demonstrated a significant growth inhibiting effect against chlorequine antiprotozoal-sensitive strains such as Plasmodium vivax, Plasmodium malariae, Plasmodium ovale etc. On the other hand, another report has indicated that Dendropanax morbifera Léveille extracts showed no clear antibacterial activity against S. mutans, while inhibited 20 - 40% of Haemophilus actinomycetemcomitans, which is anaerobic bacteria and causes periodontal diseases.23 In this study, Dendropanax morbifera Léveille extracts showed antimicrobial activities against S. mutans and C. albicans. In particular, these extracts displayed a strong antifungal effect against C. albicans, even at low concentrations. Of the Dendropanax morbifera Léveille solvent fractions, the n-hexane solvent fraction was observed to show a higher degree of antibacterial activity against S. mutans compared to the other solvent fractions. It is known that green tea24 and some other plant extracts contain polyphenolic compounds, which show antibacterial activity against S. mutans.25 It has been reported that Dendropanax morbifera Léveille also contains water-soluble tannins which are polyphenolic polymers.26; tannin suppresses proliferation of S. mutans by inhibiting the synthesis of insoluble glucans via glucosyltransferase.27 The results of this study are consistent with those of another study, which demonstrated the antibiotic activity of phenolic components extracted from the n-hexane solvent fraction of Dendropanax morbifera Léveille.22
Son et al.28 reported that the butanol and ethyl acetate solvent fractions of medicinal plants possessed strong antifungal activities against C. albicans. The analyses performed in this study also indicated the strong antifungal activity of the butanol solvent fraction highest against C. albicans. Dendropanax morbifera Léveille contains a high amount of saponin, which can be extracted using the butanol solvent. This is also consistent with previous results,29 where most antifungal agents affected a change in cell membrane permeability, leading to the leakage of fungal components, which inhibited fungal proliferation. Similarly, saponin affected the synthesis of ergosterol, a major component of C. albicans cell membranes, in order to exert an antifungal effect.29
The DPPH scavenging activity was used in this study as an index to evaluate the antioxidant effects, which lead to the improvement or prevention of oxidative stress caused by the production of active oxygen species during cellular metabolic processes. The effects were compared against those of two positive controls: vitamin C, a natural antioxidant, and BHT, a synthetic antioxidant. In this study, the Dendropanax morbifera Léveille extracts showed a higher effect (82.92 ± 0.49%) at a concentration of 500 µg/mL compared to BHT (56.71 ± 6.34%), which is consistent with the results of other studies, where the Dendropanax morbifera Léveille extracts exhibited superior antioxidant capacities. 92130
The antioxidant activity of natural extracts can be attributed to various components, and is mostly related to the total phenolic and total flavonoid content present in the extracts.30 In this study, the ethyl acetate and butanol fractions displayed superior antioxidant activities. Previous studies conducted with other natural products, such as sowthistle (Ixeris dentate Nakai),31 Dahurian rose (Rosa davurica Pall.),32 and balloon flower (Pladycodon grandiflorum),33 also exhibited the high antioxidant activities of the ethyl acetate and butanol solvent fractions. The ethyl acetate fraction of Dendropanax morbifera Léveille, in particular, contains a high amount of catethin; therefore, it showed high antioxidant activity.32 In addition, it appeared that the vitamin C and water-soluble tannin components26 in the extracts also contributed to the antioxidant activity.
As denture cleaning solutions are in direct contact with the oral mucosal tissues in the mouth, it is extremely important to determine their cytotoxic capacity. Previous studies investigating the toxicity of mouthwash solutions reported that 100 µg/mL chlorhexidine caused cell lysis,26 while 25 µg/mL of chlorhexidine inhibited epithelial cell growth.34 This study used the MTT assay in order to evaluate cytotoxicity. This is a quantitative cytotoxicity assay mainly used as a primary test for cytotoxicity.35 In general, natural extracts are known to show a lower cytotoxicity compared to synthetic compounds. Most of the studies investigating the cytotoxicity of Dendropanax morbifera Léveille have focused on its anticancer activity against cancer cells8; very few studies have attempted to investigate the cytotoxicity of Dendropanax morbifera Léveille against HNOK. The cytotoxicity assay conducted in this study showed that the Dendropanax morbifera Léveille extracts retained high cell viability (> 60%) at all concentrations. The cytotoxicity test performed using Human keratinocyte cells in a previous study revealed that the Dendropanax morbifera Léveille leaf extracts exhibited no cytotoxicity at concentrations lower than 50 µm/mL.36 This result was distinct from the results obtained using HNOK cells in this study; however, this is believed to be a result of the difference in cell sensitivities.
This study attempted to identify the antibiotic, antifungal, and antioxidant activities of Dendropanax morbifera Léveille bough extracts and solvent fractions. The results of this study confirmed the functionality of Dendropanax morbifera Léveille, as a natural product that can be used in the formulation of mouthwash and denture cleaning solutions.
Dendropanax morbifera Léveille extract turned out to have antimicrobial, antioxidant activity and cytophilicity. Based on these results, it is expected that Dendropanax morbifera Léveille extracts could be applied for the development of cost-effective and safe mouthwash and denture cleaning solutions in future.
References
1. Glass RT, Conrad RS, Bullard JW, Goodson LB, Mehta N, Lech SJ, Loewy ZG. Evaluation of microbial flora found in previously worn prostheses from the Northeast and Southwest regions of the United States. J Prosthet Dent. 2010; 103:384–389. PMID: 20493328.

2. Budtz-Jörgensen E. The significance of Candida albicans in denture stomatitis. Scand J Dent Res. 1974; 82:151–190. PMID: 4598186.

3. Baena-Monroy T, Moreno-Maldonado V, Franco-Martínez F, Aldape-Barrios B, Quindós G, Sánchez-Vargas LO. Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med Oral Patol Oral Cir Bucal. 2005; 10:E27–E39. PMID: 15800465.
4. Campos MS, Marchini L, Bernardes LA, Paulino LC, Nobrega FG. Biofilm microbial communities of denture stomatitis. Oral Microbiol Immunol. 2008; 23:419–424. PMID: 18793366.

5. Ramage G, Tomsett K, Wickes BL, López-Ribot JL, Redding SW. Denture stomatitis: a role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 98:53–59. PMID: 15243471.

6. Caton JG, Blieden TM, Lowenguth RA, Frantz BJ, Wagener CJ, Doblin JM, Stein SH, Proskin HM. Comparison between mechanical cleaning and an antimicrobial rinse for the treatment and prevention of interdental gingivitis. J Clin Periodontol. 1993; 20:172–178. PMID: 8450082.

7. Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003; 66:1022–1037. PMID: 12880330.

8. Lee SH, Park YS, Hwang B, Kim JH, Lee HY. Screening of immune activation activities in the Leaves of Dendropanax morbifera Lev. Korean J Med Crop Sci. 2002; 10:109–115.
9. Hyun TK, Kim MO, Lee H, Kim Y, Kim E, Kim JS. Evaluation of anti-oxidant and anti-cancer properties of Dendropanax morbifera Léveille. Food Chem. 2013; 141:1947–1955. PMID: 23870914.

10. Moon HI. Antidiabetic effects of dendropanoxide from leaves of Dendropanax morbifera Leveille in normal and streptozotocin-induced diabetic rats. Hum Exp Toxicol. 2011; 30:870–875. PMID: 20716587.

11. Chung IM, Kim MY, Park WH, Moon HI. Antiatherogenic activity of Dendropanax morbifera essential oil in rats. Pharmazie. 2009; 64:547–549. PMID: 19746846.
12. Chung IM, Song HK, Kim SJ, Moon HI. Anticomplement activity of polyacetylenes from leaves of Dendropanax morbifera Leveille. Phytother Res. 2011; 25:784–786. PMID: 21520473.
13. Lee CK, Kim H, Moon KH, Shin KH. Screening and isolation of antibiotic resistance inhibitors from herb materials-resistance inhibition of volatile components of Korean aromatic herbs. Arch Pharm Res. 1998; 21:62–66. PMID: 9875516.

14. Park YK, Lee WY, Ahn JK. Current review on the study of antioxidants developments from forest resources. Trend Agric Life Sci. 2006; 4:1–13.
15. Wikins TD, Holdeman LV, Abramson IJ, Moore WE. Standardized single-disc method for antibiotic susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1972; 1:451–459. PMID: 4680809.

16. Brand-Williams W, Cuvelier M, Berset C. Use of a free radical method to evaluate antioxidant activity. Technol. 1995; 28:25–30.

17. Pratten J, Smith AW, Wilson M. Response of single species biofilms and microcosm dental plaques to pulsing with chlorhexidine. J Antimicrob Chemother. 1998; 42:453–459. PMID: 9818743.

18. Gjermo P. Chlorhexidine and related-compounds. J Dent Res. 1989; 68:1602–1608.
19. Kim H, Song MJ. Analysis and recordings of orally transmitted knowledge about medicinal plants in the southern mountainous region of Korea. J Ethnopharmacol. 2011; 134:676–696. PMID: 21256949.

20. Islam B, Khan SN, Khan AU. Dental caries: from infection to prevention. Med Sci Monit. 2007; 13:RA196–RA203. PMID: 17968308.
21. Kulak Y, Arikan A, Kazazoglu E. Existence of Candida albicans and microorganisms in denture stomatitis patients. J Oral Rehabil. 1997; 24:788–790. PMID: 9372471.

22. Im GP, Jeong HJ, Lee JS. Studies on the Technical Improvement to use "Hwangchil-traditional Korean golden varnish" and the diversified uses of Dendropanax morbiera Lev.2nd Report. Korea: Korean Ministry of Science and Technology;1996. p. 1–257.
23. Baek DH. Screening of the natural plant extracts for the antimicrobial activity on dental pathogens. Korean J Microbiol. 2007; 43:227–231.
24. Otake S, Makimura M, Kuroki T, Nishihara Y, Hirasawa M. Anticaries effects of polyphenolic compounds from Japanese green tea. Caries Res. 1991; 25:438–443. PMID: 1667297.

25. Smullen J, Koutsou GA, Foster HA, Zumbé A, Storey DM. The antibacterial activity of plant extracts containing polyphenols against Streptococcus mutans. Caries Res. 2007; 41:342–349. PMID: 17713333.
26. Kim HR, Chung HJ. Chemical characteristics of the leaves and the seeds of Korean Dendropanax (Dendropanax morbifera Lev.). J Korean Soc Appl Biol Chem. 2000; 43:63–66.
27. Yanagida A, Kanda T, Tanabe M, Matsudaira F, Oliveira Cordeiro JG. Inhibitory effects of apple polyphenols and related compounds on cariogenic factors of mutans streptococci. J Agric Food Chem. 2000; 48:5666–5671. PMID: 11087536.

28. Son HY, Kim EJ, Gwon YS, Gwon GS, Jin IR, Gwon HY, Gwon JS, Son GH. Screening of anti-candidiosis agent from medicinal and wild plants. J Life Sci. 2003; 13:604–617.
29. Coleman JJ, Okoli I, Tegos GP, Holson EB, Wagner FF, Hamblin MR, Mylonakis E. Characterization of plant-derived saponin natural products against Candida albicans. ACS Chem Biol. 2010; 5:321–332. PMID: 20099897.

30. Zou Y, Liao S, Shen W, Liu F, Tang C, Chen CY, Sun Y. Phenolics and antioxidant activity of mulberry leaves depend on cultivar and harvest month in Southern China. Int J Mol Sci. 2012; 13:16544–16553. PMID: 23443117.

31. Hong S, Jeong D, Kim K, Hwang E. The composition of the root of Ixeris dentate var. albiflora Nakai. and cell viability and DPPH radical scavenging activities of tist extract. Korean J Nutr. 2010; 43:105–113.
32. Shin IC, Jeong KJ, Shim TH, Oh HS, Park SK, Cheung EH, Kim SN, Kim GG, Choi DS, Kwon YS, Kim CM, Sa JH. Catechin content and antioxidative effect from Rosa davurica Pall. Korean J Pharmacogn. 2002; 33:177–181.
33. Jang JR, Hwang SY, Lim SY. Inhibitory effect of extracts of Platycodon grandiflorum (the ballon flower) on oxidation and nitric oxide production. Korean J Food Preserv. 2011; 18:65–71.

34. Helgeland K, Heyden G, Rölla G. Effect of chlorhexidine on animal cells in vitro. Scand J Dent Res. 1971; 79:209–215. PMID: 4105986.

35. Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987; 47:936–942. PMID: 3802100.
Fig. 1
Antibacterial and antifungal activity of Dendropanax morbifera Léveille extract against (A) S.mutans and (B) C. albicans (C; Control, 20; 20 µg/mL, 40; 40 µg/mL, 80; 80 µg/mL, 100; 100 µg/mL).
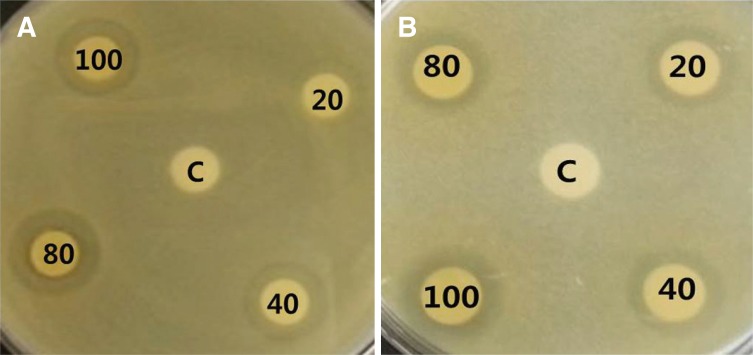
Fig. 2
Antibacterial activity of the Dendropanax morbifera Léveille solvent fraction against S. mutans (C; Control, 20; 20 µg/mL, 40; 40 µg/mL, 80; 80 µg/mL, 100; 100 µg/mL). (A) n-hexane, (B) ethyl acetate, (C) chloroform, (D) butanol.
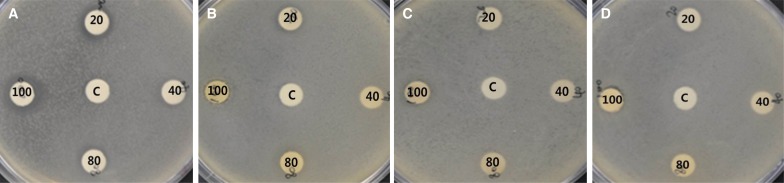
Fig. 3
Antifungal activity of the Dendropanax morbifera Léveille solvent fraction against C. albicans (C; Control, 20; 20 µg/mL, 40; 40 µg/mL, 80; 80 µg/mL, 100; 100 µg/mL). (A) n-hexane, (B) ethyl acetate, (C) chloroform, (D) butanol.
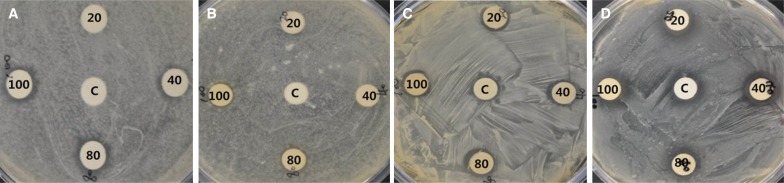
Table 1
Inhibition zone of Dendropanax morbifera Léveille extract against S. mutans and C. albicans
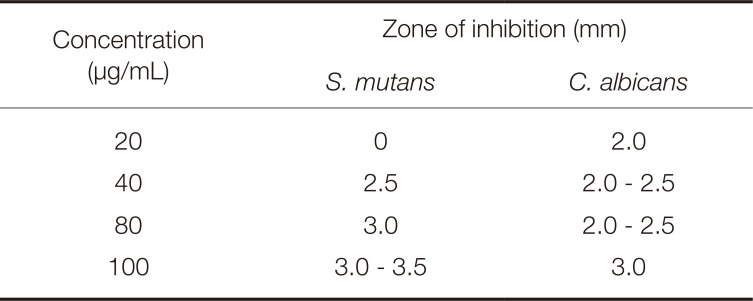
| Concentration (µg/mL) | Zone of inhibition (mm) | |
|---|---|---|
| S. mutans | C. albicans | |
| 20 | 0 | 2.0 |
| 40 | 2.5 | 2.0 - 2.5 |
| 80 | 3.0 | 2.0 - 2.5 |
| 100 | 3.0 - 3.5 | 3.0 |
Table 2
Inhibition zone (mm) of the Dendropanax morbifera Léveille solvent fraction against S. mutans
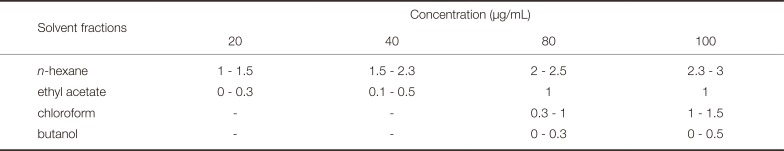
| Solvent fractions | Concentration (µg/mL) | |||
|---|---|---|---|---|
| 20 | 40 | 80 | 100 | |
| n-hexane | 1 - 1.5 | 1.5 - 2.3 | 2 - 2.5 | 2.3 - 3 |
| ethyl acetate | 0 - 0.3 | 0.1 - 0.5 | 1 | 1 |
| chloroform | - | - | 0.3 - 1 | 1 - 1.5 |
| butanol | - | - | 0 - 0.3 | 0 - 0.5 |
Table 3
Inhibition zone (mm) of the Dendropanax morbifera Léveille solvent fraction against C. albicans
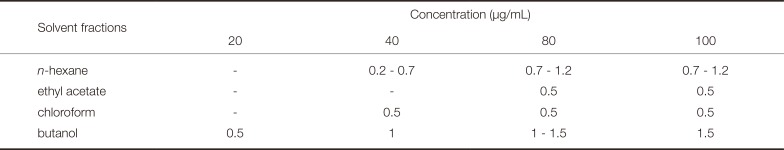
| Solvent fractions | Concentration (µg/mL) | |||
|---|---|---|---|---|
| 20 | 40 | 80 | 100 | |
| n-hexane | - | 0.2 - 0.7 | 0.7 - 1.2 | 0.7 - 1.2 |
| ethyl acetate | - | - | 0.5 | 0.5 |
| chloroform | - | 0.5 | 0.5 | 0.5 |
| butanol | 0.5 | 1 | 1 - 1.5 | 1.5 |
Table 4
DPPH radical scavenging activity (%) of the Dendropanax morbifera Léveille extracts
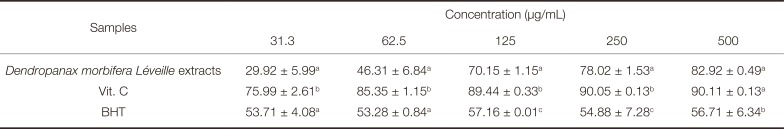
Table 5
DPPH radical scavenging activity (%) of the Dendropanax morbifera Léveille solvent fraction




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download