Abstract
PURPOSE
The purpose of this study was to compare the removal torques of a chemically modified SLActive implant and a blasted, laser-treated (BLT) implant, which were soaked in saline for 2 weeks after their surface modifications. The removal torques of the two implants were measured 4 weeks after their implantation into the bone defect area in rabbit tibias with concentrated growth factor (CGF) application.
MATERIALS AND METHODS
To make artificial bone defects in the cortical layers of both tibias, an 8-mm diameter trephine bur was used. Then, prepared CGF was applied to the bony defect of the left tibia, and the bony defect of the right tibia was left unfilled. Four weeks later, the surgical sites of 16 rabbits were re-exposed. For 8 rabbits, the SLActive implants (Straumann, Switzerland) were inserted in the left tibia, and the BLT implants (CSM implant, Daegu, Korea) were inserted in the right tibia. For other rabbits, the BLT implants were inserted in the left tibia, and the SLActive implants were inserted in the right. Four weeks afger the insertion, torque removal was measured from 4 rabbits exterminated via CO2 inhalation.
Since implant treatment is becoming more common in the field of dentistry, there has been an increase in cases requiring bone formation through bone regeneration in the alveolar bone defect area at the site of the implant. Sufficient bone level in the edentulous area is a prerequisite for the successful placement of the dental implant. Platelet-rich plasma (PRP) was first introduced by Marx in 1998. PRP collected from a centrifuge was shown to have 333% increased platelet level compared to that in venous blood. In addition, it is reported that PRP accelerates ossification in patients with edentulous mandible. Calcium chloride and bovine thrombin mixture is typically used in the preparation process of PRP.1
Platelet-rich fibrin (PRF) was introduced by Choukroun in 2006. Compared to PRP, platelet concentrate from blood can be obtained without any biochemical manipulation. It induces effective cell migration, proliferation, and healing.2 Cho et al.3 proposed higher implant removal torque in bone defect area healed with PRF compare to that without PRF. From the result, it was found that PRF had a positive influence on bone formation. Su et al.4 performed quantitative analysis of growth factor in PRF release and supernatant serum as well.
Concentrated-growth factor (CGF) was first introduced by Sacco in 2011; Sacco showed that gelatin platelet can be obtained using a centrifuge. The study revealed an abundant level of VEGF, TGF-β1, and CD34 in CGF.5 Many studies about bone formation inducement on the bone defect area using PRP, PRF, and CGF are in progress; however, the most effective solution has yet to be determined.67891011
Surface treatment of implants is another popular area in current research. According to the current knowledge, laser surface treatment has been shown to promote excellent osseointegration by increasing the hardness, corrosion resistance, surface property, and purity.1213 Cho et al.14 compared the removal torque between a machined implant and laser-treated implant in a rabbit tibia. They found that the removal torque of the laser-treated implant was 2.5 times as much as that of the machined implant.14 Furthermore, according to Buser et al., resorbable blast media (RBM) treatment, hydroxyapatite (HA) coating, titanium plasma spray, acid etching, and sand blasting treatment increase surface roughness of implant, which leads to the increase in bone-to-implant contact (BIC) and cell adhesion.1516
Buser et al.17 compared the SLActive implant (Straumann, Switzerland) soaked in saline and SLA (sandblasted, large-grit, acid-etched) 2 weeks after implantation into a minipig. They found that the BIC of the SLActive implant was 60% higher than that of SLA. Another study revealed that surface treatment of the SLActive implant (Institut Straumann AG, Basel, Switzerland) resulted in 162% higher fibronectin adhesion rate than the SLA implant.18 Storage of a surface-treated implant in saline enhances the surface-free energy and hydrophilicity of the implant. It is known that the SLActive implant surface is exceptionally hydrophilic with a contact angle of almost 0° while that of SLA is 139.0°.19
The purpose of the present study was to evaluate and compare the removal torque of Straumann's SLActive implant and the designed implant with blasting and laser surface treatment two weeks after implantation into bone defects area in rabbit tibia. CGF was applied to the bone defect area to promote healing, and implants used in the study were stored in saline for two weeks before implantation.
CGF was prepared by sampling 2 mL of venous blood from a rabbit ear. The blood sample was centrifuged for about 12 min in the speed range of 2400 - 2700 rpm in a Medifuse machine (Silfradent S.R.L., Sofia, Italy).
The resultant solution after centrifugation was separated into 4 layers. The top layer was the acellular plasma layer. CGF, which was aggregated in the middle layer, was prepared by cutting the lower layer (red corpuscle layer) with a scissor. Then, the CGF layer was applied to the rabbit's bone defect area.
Sixteen commercial SLActive implants (3.3 mm in diameter, 8 mm in length) were selected for use. Sixteen additional titanium implants with the same design were prepared (CSM implant; CSM company, Daegu, Korea); then, they were blasted and laser treated. Machined titanium implant surfaces were treated with an Nd:YAG laser (Jenoptic AG, Jena, Germany) with a 15 kHz wave-length, 10 W rated output, and pulse width of 2 usec. The surface-treated implants were soaked in saline for more than 2 weeks until insertion.
Twenty-four adult New Zealand white rabbits were used in this experiment. This study was approved by the Animal Care and Use Committee of Kyungpook National University (KNU 2014-0001-2). The mean weight of the rabbits was 3.0 kg before the experiment and 3.7 kg at sacrifice. Intramuscular injection of Tiletamine/Zolazepam (Zoletil 50; Virbac Laboratories, France; 0.2 mL/kg) was used for anesthesia, and 2% lidocaine was injected into the surgical site for local anesthesia.
After anesthesia, the hair of the rabbit was removed, and the skin was disinfected with iodine and 75% alcohol. An incision was made with a #15 blade, and the bone was exposed with a periosteal elevator. To make the artificial defect in both tibias, an 8-mm trephine bur was used for implant insertion, and bone removal was conducted at 1200 rpm.
Prepared CGF was applied to the bony defect of the left tibia, and the right bony defect was left unfilled. Prior to skin suture, a periosteal suture was performed using 4-0 vicryl.
Four weeks after the first surgery, re-incision was made on the surgical site to expose the bone again. Installation of the blasted and laser-treated (BLT) implants was performed on the experimental group while the control group had SLA implants installed.
The experiment was performed independently on four groups and designed to test the effect of CGF and surface treatment of the implant. In group A, BLT implant was installed on CGF applied bone defect. In group B, BLT implant was installed on unfilled bone defect. In group C, SLActive implant was installed on CGF applied bone defect. In group D, SLActive implant was installed on unfilled bone defect (Fig. 1).
All the implants inserted on the bone defect area were 3.3 mm in diameter and 8 mm in depth. Each implant was inserted monocortically and did not engage the opposite cortical bone. Drilling was performed at 800 rpm with a 3.0-mm diameter drill. Counter-sinking was omitted during installation. The insertion torque of every implant was measured using a digital torque gauge (MGT-12 digital torque gauge; Mark-10 Corp., New York, NY, USA) during the procedure. One milliliter of each analgesic, antibiotics, and metabolism accelerator was given by intramuscular injection once a day for a week. Additionally, we avoided placing any stress on the rabbits as stress might influence the test result. Four weeks later, all the rabbits were sacrificed with CO2 and the removal torque of the inserted implant was measured.
Another incision on the rabbit's leg was made to expose the inserted implant in order to measure the removal torque using a torque-measuring device (MGT-12 digital torque gauge, Mark-10 Corp., New York, NY, USA). To remove the implant, torque was applied in a counterclockwise direction. Then, the maximum removal torque was measured in N/cm.
A Student's t-test was performed for statistical analysis of insertion and removal torque with statistical significance set at 0.05. PASW 17.0 (SPSS Inc., Chicago, IL, USA) was used for torque measurement analysis.
Insertion of the implants revealed a mean torque of 4.1 N in group A, 4.9 N in group B, 3.5 N in group C, and 5.8 N in group D (Table 1). The t-test showed no significant differences among all groups. No significant differences were observed based on CGF application and the type of fixture used in the experiment (Fig. 2).
The implants were removed with a mean torque of 36.6 N in group A, 41.7 N in group B, 39.8 N in group C, and 42.9 N in group D (Table 2). To find statistical difference, P value among the groups were calculated. P values were 0.666 between group A and B, 0.598 between group C and D, 0.647 between A and C, and 0.886 between B and D. No significant differences were observed among groups; hence, the BLT implant and SLActive implant are deemed to have similar removal torques (Fig. 3).
In this study, we compared the insertion torque measurements of BLT implants and SLActive implants placed on bony defect sites with and without CGF application. Our results supported that 4 weeks of healing allowed adequate time for remodeling on the bone defect area in a rabbit model. However, insertion torque measurements were inappropriate for bone healing assessment because the highly variable measurements depended on the surgical technique. Since this study showed similar insertion torque measurements of implants in all groups, we deemed that the insertion torque did not affect removal torque measurements.
Alissa et al. demonstrated the positive influence of PRP on tissue healing of an extraction socket.20 On the other hand, Arenaz-Búa et al.21 pointed out that PRP application in a third molar extraction socket did not show any synergistic activity in bone regeneration after 6 months. Additionally, no significant difference could be found with respect to pain and edema. Moreover, Gübüzer et al.22 reported that the application of PRP in a third molar extraction socket did not enhance osteoblastic activity. According to the review paper by Albanese et al.,23 application of PRP to the alveolar socket does enhance the soft tissue healing while its effectiveness on bone regeneration is yet to be prove. The effectiveness of PRP is still controversial because although the application of PRP influences the early phase of the bone healing process, its effects fade quickly after a few days.23
Garcia et al.24 showed that applying PRP accelerated soft tissue regeneration and peri-implant bone repair. Daif proposed that direct application of PRP on a fracture line of mandible did improve bone regeneration.25 A similar research by Poeschl showed better outcomes in maxillary sinus lifts, which were achieved by applying PRP mixed with graft material on maxillary sinus augmentation.26 Su et al. proposed that the levels of PRF, PDGF-AB, TGF-β1, VEGF, EGF, and IGF-1 are high PRF releasate and acellular plasma membrane in clinical applications, and PRF should be applied as soon as possible into the surgical site for best clinical results. Cho et al.27 showed high removal torque of an implant inserted in a bony defect area along with the application of CGF and tooth ash. However, Esposito et al.28 stated that using PRP with autogenous bone or bone substitute did not show any clinical effects on sinus lift. Overall, the scientific evidence on the effect of PRF is insufficient and remains controversial at this time.
In this study, the removal torque was not influenced by use of CGF. We, thus, assumed that CGF was not effective since there was no statistical difference in removal torque between the CGF applied groups and the group without CGF. CGF releases growth factor only for the first few days of an 8-week healing period. Therefore, removal torque is not influenced during the long-term healing period. Laser irradiation on the titanium implant surface forms pores that are 25 µm in diameter and 25 µm in depth. Distance between the pores is 10 - 12 µm.14 Mustafa et al. reported that enhanced roughness of titanium implant accelerated the differentiation and proliferation of cells in the mandible.29 As previously discussed, the results of removal torque measurements of the designed implant with a BLT surface were similar to that of the SLActive implant, which is known to be the gold standard surface treatment. This fact supports that the BLT method may serve as a potential treatment that is as effective as the gold standard.
Furthermore, by soaking the surface-treated implant in saline for more than 2 weeks, the surface changed from hydrophobic to hydrophilic, which shortened the time of fixation and increased the initial stability of implants. This result is similar to the result by the study of Rupp et al..30 Storing a surface-treated implant in saline promotes rapid osseointegration by increasing the hydrophilicity. In addition, it allows for immediate loading by shortening the time for osseointegration of the implant after insertion.
Further study may be needed to clarify the influence of various storage solutions on osseointegration of surface-treated implants.
Though there were certain limitations in this study, several important findings were obtained. First, CGF application was irrelevant to both insertion and removal torque of the implant. Thus, CGF application to bone defect area appeared to be ineffective in bone regeneration. Second, our findings showed that both types of implants had similar removal torque values, thus providing evidence that BLT implants could be as effective as the gold standard SLActive implant. Finally, fixtures soaked in saline after BLT surface modification exhibited excellent osseointegration.
Figures and Tables
Fig. 1
In group A, BLT implant was installed on CGF applied bone defect. In group B, BLT implant was installed on non-CGF applied bone defect. In group C, SLActive implant was installed on CGF applied bone defect. In group D, SLActive implant was installed on non-CGF applied bone defect.
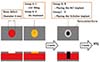
Table 1
Insertion torque

Table 2
Removal torque

(Horizontal line means groups. In group A, BLT implant was installed on CGF applied bone defect. In group B, BLT implant was installed on unfilled bone defect. In group C, SLActive implant was installed on CGF applied bone defect. In group D, SLActive implant was installed on unfilled bone defect. Vertical line means rabbit numbers.)
References
1. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 85:638–646.
2. Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101:e37–e44.
3. Cho SA, Lee BK, Park SH, Ahn JJ. The bone integration effects of platelet-rich fibrin by removal torque of titanium screw in rabbit tibia. Platelets. 2014; 25:562–566.
4. Su CY, Kuo YP, Tseng YH, Su CH, Burnouf T. In vitro release of growth factors from platelet-rich fibrin (PRF): a proposal to optimize the clinical applications of PRF. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 108:56–61.
5. Rodella LF, Favero G, Boninsegna R, Buffoli B, Labanca M, Scarì G, Sacco L, Batani T, Rezzani R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc Res Tech. 2011; 74:772–777.
6. Aghaloo TL, Moy PK, Freymiller EG. Evaluation of platelet-rich plasma in combination with anorganic bovine bone in the rabbit cranium: a pilot study. Int J Oral Maxillofac Implants. 2004; 19:59–65.
7. Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999; 14:529–535.
8. Froum SJ, Wallace SS, Tarnow DP, Cho SC. Effect of platelet-rich plasma on bone growth and osseointegration in human maxillary sinus grafts: three bilateral case reports. Int J Periodontics Restorative Dent. 2002; 22:45–53.
9. Mazor Z, Peleg M, Garg AK, Luboshitz J. Platelet-rich plasma for bone graft enhancement in sinus floor augmentation with simultaneous implant placement: patient series study. Implant Dent. 2004; 13:65–72.
10. Whitman DH, Berry RL, Green DM. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997; 55:1294–1299.
11. Wiltfang J, Kloss FR, Kessler P, Nkenke E, Schultze-Mosgau S, Zimmermann R, Schlegel KA. Effects of platelet-rich plasma on bone healing in combination with autogenous bone and bone substitutes in critical-size defects. An animal experiment. Clin Oral Implants Res. 2004; 15:187–193.
12. Picraux ST, Pope LE. Tailored surface modification by ion implantation and laser treatment. Science. 1984; 226:615–622.
13. Gaggl A, Schultes G, Müller WD, Kärcher H. Scanning electron microscopical analysis of laser-treated titanium implant surfaces--a comparative study. Biomaterials. 2000; 21:1067–1073.
14. Cho SA, Jung SK. A removal torque of the laser-treated titanium implants in rabbit tibia. Biomaterials. 2003; 24:4859–4863.
15. Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991; 25:889–902.
16. Ahn SJ, Leesungbok R, Lee SW. Histomorphometric analysis and removal torque of small diameter implants with alternative surface treatments and different designs. J Oral Implantol. 2010; 36:263–272.
17. Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, Hoffmann B, Lussi A, Steinemann SG. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004; 83:529–533.
18. Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, Boyan BD. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005; 74:49–58.
19. Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006; 76:323–334.
20. Alissa R, Esposito M, Horner K, Oliver R. The influence of platelet-rich plasma on the healing of extraction sockets: an explorative randomised clinical trial. Eur J Oral Implantol. 2010; 3:121–134.
21. Arenaz-Búa J, Luaces-Rey R, Sironvalle-Soliva S, Otero-Rico A, Charro-Huerga E, Patiño-Seijas B, García-Rozado A, Ferreras-Granados J, Vázquez-Mahía I, Lorenzo-Franco F, Martín-Sastre R, López-Cedrún JL. A comparative study of platelet-rich plasma, hydroxyapatite, demineralized bone matrix and autologous bone to promote bone regeneration after mandibular impacted third molar extraction. Med Oral Patol Oral Cir Bucal. 2010; 15:e483–e489.
22. Gürbüzer B, Pikdöken L, Urhan M, Süer BT, Narin Y. Scintigraphic evaluation of early osteoblastic activity in extraction sockets treated with platelet-rich plasma. J Oral Maxillofac Surg. 2008; 66:2454–2460.
23. Albanese A, Licata ME, Polizzi B, Campisi G. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing. 2013; 10:23.
24. Garcia RV, Gabrielli MA, Hochuli-Vieira E, Spolidorio LC, Filho JG, Neto FA, de Cardoso LA, Shibli JA. Effect of platelet-rich plasma on peri-implant bone repair: a histologic study in dogs. J Oral Implantol. 2010; 36:281–290.
25. Daif ET. Effect of autologous platelet-rich plasma on bone regeneration in mandibular fractures. Dent Traumatol. 2013; 29:399–403.
26. Poeschl PW, Ziya-Ghazvini F, Schicho K, Buchta C, Moser D, Seemann R, Ewers R, Schopper C. Application of platelet-rich plasma for enhanced bone regeneration in grafted sinus. J Oral Maxillofac Surg. 2012; 70:657–664.
27. Jeong IY, Cho SA. The bone healing effects of concentrated growth factors with a tooth ash by removal torque of titanium screw in rabbit tibia. Archives of Oral Biology (In review).
28. Esposito M, Grusovin MG, Rees J, Karasoulos D, Felice P, Alissa R, Worthington HV, Coulthard P. Interventions for replacing missing teeth: augmentation procedures of the maxillary sinus. Cochrane Database Syst Rev. 2010; (3):CD008397.
29. Mustafa K, Wennerberg A, Wroblewski J, Hultenby K, Lopez BS, Arvidson K. Determining optimal surface roughness of TiO(2) blasted titanium implant material for attachment, proliferation and differentiation of cells derived from human mandibular alveolar bone. Clin Oral Implants Res. 2001; 12:515–525.
30. Rupp F, Scheideler L, Eichler M, Geis-Gerstorfer J. Wetting behavior of dental implants. Int J Oral Maxillofac Implants. 2011; 26:1256–1266.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download