Abstract
PURPOSE
This study was performed to characterize the effects of zirconia coated with calcium phosphate and hydroxyapatite compared to smooth zirconia after bone marrow-derived osteoblast culture.
MATERIALS AND METHODS
Bone marrow-derived osteoblasts were cultured on (1) smooth zirconia, (2) zirconia coated with calcium phosphate (CaP), and (3) zirconia coated with hydroxyapatite (HA). The tetrazolium-based colorimetric assay (MTT test) was used for cell proliferation evaluation. Scanning electron microscopy (SEM) and alkaline phosphatase (ALP) activity was measured to evaluate the cellular morphology and differentiation rate. X-ray photoelectron spectroscopy (XPS) was employed for the analysis of surface chemistry. The genetic expression of the osteoblasts and dissolution behavior of the coatings were observed. Assessment of the significance level of the differences between the groups was done with analysis of variance (ANOVA).
RESULTS
From the MTT assay, no significant difference between smooth and surface coated zirconia was found (P>.05). From the SEM image, cells on all three groups of discs were sporadically triangular or spread out in shape with formation of filopodia. From the ALP activity assay, the optical density of osteoblasts on smooth zirconia discs was higher than that on surface treated zirconia discs (P>.05). Most of the genes related to cell adhesion showed similar expression level between smooth and surface treated zirconia. The dissolution rate was higher with CaP than HA coating.
Commercially pure titanium has been employed as the best material for dental implants due to its biocompatibility and excellent mechanical properties. Healing of peri-implant tissue can be influenced by the physicochemical and mechanical properties of the implant material, microstructures, macrostructures, and surface chemistry of the implant. The surface topography and chemistry of an implant material can have beneficial or disadvantageous influence on cell adhesion and proliferation, thereby controlling the osseointegration process. Studies on the effects of surface modified materials on the adherence and spreading of cells have recently been reported or are in progress.1,2,3,4,5
One of the handicaps of titanium from an esthetic point of view, as a dental implant material, is that the dark gray color of titanium can shine through the thin soft tissues. Soft tissue shrinkage leading to gingival recession or peri-implantitis may leave the cervical titanium component visible. Implants or transgingival abutments from tooth-colored materials such as zirconia ceramic may be one possible solution to these problems with the dark color of titanium. Zirconia has tooth-like ivory color and somewhat translucency, making it sufficient material for esthetic restorations.
Zirconia ceramic is a biocompatible material that has optimal esthetic and mechanical properties for dental implants. The biomaterial-related properties of zirconia are as advantageous as those of titanium.6 Tissue reaction and stability of zirconia, which are important factors in maintaining zirconia restorations free of periodontal problems, proved to be satisfactory.7,8 Bacterial coverage and accumulation on zirconia was reported to be lower than on titanium.9,10 Inflammation associated processes in peri-implant soft tissues were found to be higher around titanium than around zirconia.11 The material composition and profile of transgingival implant components seems to influence cell behavior and growth. The macrostructures of the zirconia ceramic are able to provide contact guidance and gingival contouring to provide biological seal.1,12 Zirconia ceramic can be suitable for transgingival implant components13 resulting in final esthetic results, but more clinical and mechanical trials are necessary for a complete understanding of the behavior of zirconia abutments and implants over a long time period.
In hard tissue engineering, calcium phosphate (CaP) ceramics, such as hydroxyapatite [HA; Ca10(PO4)6(OH)2] and tricalcium phosphate [TCP, Ca3(PO4)2], have attracted attention due to their excellent biocompatibility and osteoconductivity.14 Clinical reports on the CaP ceramics proved their direct bonding to bone and complete osseointegration. However, their poor mechanical properties, such as low strength and fracture toughness, limited wide application in hard tissue implants. Calcium phosphate was accumulated on zirconia surfaces by ion beam assisted deposition (IBAD) and hydroxyapatite by aerosol deposition. The compositional degradation of calcium phosphate coatings is caused by the deposition process involving very high temperatures, low binding strength, and thick coatings. The binding strength between the coating and the implant material is one of the critical characteristics which affect the long-term stability of the bone-implant interface.
The cell culture system used in this study was rat bone marrow-derived osteoblasts. After implantation, the surface properties of biomaterials can affect the osteogenic and differentiation potentials of mesenchymal cells.
This study was performed to characterize the attachment and growth behavior of bone marrow-derived osteoblasts cultured on zirconia surfaces with calcium phosphate coatings and hydroxyapatite coatings compared to smooth surfaced zirconia. Also, we evaluated the dissolution rates of the coatings of surface treated zirconia.
Zirconia discs (LAVA™, 3M ESPE, St. Paul, MN, USA) of Y-TZP (yttrium-stabilized tetragonal zirconia polycrystal) with a diameter of 10 mm and a thickness of 2 mm were prepared by pressing and sintering at 1500℃ for 2 hours. The zirconia discs of three surface types were prepared. One type was Y-TZP with a smooth surface (ZS group), another was Y-TZP with IBAD (Ion Beam Assisted Deposition) Ca-P coating (CaP group), the third was Y-TZP with HA deposition (HA group). Twenty discs of each group were fabricated and tested for proliferation, differentiation, osteogenic potential, and gene expression. Disc samples were cleaned ultrasonically in acetone, ethanol, and de-ionized water.
Calcium phosphate film (up to 500 nm) was deposited on zirconia disk by the electron-beam deposition system. Mixed powder of hydroxyapatite (Alfa Aesar, Johnson Matthey, London, UK) and calcium oxide (Sigma, St. Louis, MO, USA) were sintered at 1000℃ for 2 hours. An electron beam evaporator (Telemark, Battle Ground, WA, USA) at 7.5 kV and 0.13 A, and an end-hall type ion gun (Ionbeam Scientific, Berks, UK) at 90 V and 2.0 A were employed for deposition. Heat treatment after the deposition was conducted at 400℃ in the vacuum of 3 mm Torr.
The raw HA powder (Alfar Aesar, Ward Hill, MA, USA) used in this study was subjected to the pre-deposition treatment consisting of heating to 1100℃ for 1 hour. HA deposition was conducted by using an aerosol deposition system. The powder was placed in a vibrating aerosol chamber that contained fine floating particles. The fine particles were carried by oxygen gas and sprayed onto the zirconia disk in the deposition chamber. The chamber was continuously evacuated using a mechanical booster and rotary pumps.
Bone marrow-derived osteoblasts were harvested and cultured following the methods described by Maniatopoulos et al.15 Femurs of two six-week-old male Sprague-Dawley rats were removed. The bones were washed with 70% alcohol and immersed twice in alpha-MEM (Sigma, St. Louis, MO, USA) culture medium containing 100 units/mL penicillin-G, 100 g/mL streptomycin (Gibco BRL, Life Technologies BV, Bleiswijk, The Netherlands). The condyles were removed and the bone marrow flushed out using complete cell culture medium (alpha-MEM) with 15% fetal bovine serum (Gibco, Invitrogen Ltd., Paisley, UK) and supplemented with 50 µg/mL ascorbic acid (Sigma, St. Louis, MO, USA), 7 mM Na-beta-glycerophosphate (Sigma, St. Louis, MO, USA). The resulting suspension was passed through a 22-gauge needle. The adherent cell population was cultured at 37℃ in 5% CO2. After seven days of primary culture, cells were trypsinized and resuspended in complete culture medium. Cells culture on disks placed in a 24-well plate was carried out for all experiments.
Cells were collected and seeded at a density of 1 × 105 cells/mL by using 0.1% trypsin and 0.02% EDTA in Ca++ and Mg++-free Eagle's buffer for cell release. Each set of wells contained 24 sterile zirconia disks at 37℃ in 5% CO2 for 24 hours. The samples were then moved to new dishes, fresh media were added, and the plated disks were cultured at 37℃ in 5% CO2 for an additional 24 hours. All cell culture media were supplemented with 100 units/mL penicillin-G, 100 g/mL streptomycin, and 0.25 g/mL fungizone (Gemini Bio-Products Inc., Woodland, CA, USA).
The cellular viability and proliferation of cells were examined with an MTT based cell growth determination kit (CGD1; Sigma, St. Louis, MO, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoliumsalt (MTT), which turns into a blue formazan product due to the viable mitochondria in active cells was used. The osteoblasts were seeded at a density of 2 × 104 cells/mL and incubated at 37℃ in 5% CO2 for 24 hours and 48 hours. After 24 hours and 48 hours of incubation, the discs were moved to well plates and new media were added. Then, diluted MTT (5 mg/mL) solution was added, and the incubation was continued at 37℃ in 5% CO2 for 4 hours. The incubation medium was then removed, 400 µL of isopropanol with 0.04 N HCl was added to each well, and the resulting formazan crystals were dissolved. The absorbance of the formazan product at 490 nm was measured with a microplate reader (Bio-kinetics reader, EL312e, Winooski, VT, USA). The absorbance of formazan reflects the level of cell metabolism. Experiments were repeated independently in triplicate.
SEM was used to analyze the cellular attachment and morphology of the cells. Cells were seeded at a density of 2 × 104 cells/mL. The cultured cells were incubated for 24 and 48 hours at 37℃ in 5% CO2. The loosely-adherent or unbound cells were removed from the wells by aspiration. Wells were washed twice with a 0.1 M phosphate buffered saline (PBS) buffer (pH 7.4), and the remaining bound cells were fixed with 2.5 % glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.3, for more than 1 hour. The excess glutaraldehyde solution was removed and the cells were rinsed once more in PBS before being dehydrated in ethanol baths of progressively higher concentrations (50, 60, 70, 80, 90, 95, and 100%, 10 min in each bath). After the cells were dried to a critical point, the samples were sputtered with a 100 nm thick layer of gold using an ion coater (IB-3, Eiko Co., Tokyo, Japan). Attachment and morphology of the cells on the discs were observed by VEGA SEM (TESCAN, Brno, Czech Republic). Images were recorded at 5,000× magnification.
Osteoblast differentiation generally implies expression of alkaline phosphatase (ALP), specific protein and mineralization capacity. ALP is a widely used osteoblast marker, and increased ALP activity is associated with elevated osteoblastic activity. ALP activity level was evaluated after 14 days. The cells were seeded on the substrate at a density of 5 × 104 cells per well. Cells were washed twice in cold Tris-buffered saline (TBS), lysed with TBS-Triton and centrifuged, and the supernatant was analysed for ALP activity using 1 mg/mL p-nitrophenylphosphate (pNPP; Sigma, St Louis, MO, USA). The hydrolysis of pNPP into p-nitrophenol in alkaline buffer solution was quantified. The absorbance at 409 nm was measured using the microplate reader (Bio-Rad, Hercules, CA, USA). The enzyme activity was expressed as µM p-nitrophenol/µg protein.
X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition of the surface (top 0-10 nm usually).
Surface chemistry of the substrates was analyzed by x-ray photoelectron spectroscopy (XPS, K-Alpha; Thermo Fisher Scientific, MA, USA). The XPS spectra were recorded using normal Al Kα (1486.6 eV) with a probing beam size of 125 µm. The recorded spectra were calibrated to the binding energy of C 1s (284.6 eV) owing to the charge effect. XPS spectra were obtained at Zr 3d, Y 3d, C 1s, O 1s, P 2p, Ca 2p, and Al 2p.
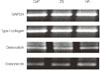
The osteoblastic differentiation was evaluated by RT-PCR for examination of type I collagen, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), osteocalcin, and osteonectin. RT-PCR was performed after 24 hours of cell incubation. Total RNA extraction was performed with the RNeasy mini kit (Qiagen, Chatsworth, CA, USA). The extracted total RNA samples were converted to cDNA. AmpliTaq DNApolymerase (Amersham Pharmacia Biotech, Piscataway, NJ, USA) was used to amplify the cDNA. The PCR products were fractionated by 1.5% agarose gel electrophoresis and visualized by ethidium bromide staining. The intensity of the bands was quantified under UV transillumination (Eagle Eye II, Stratagene, La Jolla, CA, USA).
Dissolution behavior of Ca2+ and P- ions from the coatings were evaluated by immersion of CaP and HA group specimens in physiological saline solution (0.9%) NaCl. At the predetermined time periods (4, 8, 12, 16, and 20 hours), the concentration of the Ca2+ and P- ions released from the coated substrate was calculated with inductively coupled plasma-atomic emission spectrophotometer (ICP-AES; ICPS-1001V, Shimadzu, Tokyo, Japan) analysis.
The mean values (MV) and standard deviations (SD) will be computed for the MTT test and ALP analysis, and an analysis of variance (ANOVA) and Scheffe post hoc tests for multiple comparisons were conducted to assess the statistical significance of the differences between the groups. Differences were considered significant at P<.05. All statistical analyses were performed using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
The optical densities of the formazan produced by osteoblasts in the ZS, CaP, and HA groups were measured after 4, 24 and 48 hours via a MTT assay (Fig. 1). The degree of cell proliferation for the CaP group was highest after 4 hours. After 48 hours of cell culture, all three groups showed increased cellular activity and proliferation, however, no significant differences were observed among the groups (P>.05). Overall, the osteoblasts seeded onto the three groups of zirconia specimens showed similar degrees of vitality and proliferation.
The general morphology and growth pattern of the osteoblasts for each group were observed using SEM for each group (Fig. 2 and Fig. 3). On all three groups of specimens, SEM images after 24 and 48 hours of culture show that the cells were triangular or elongated in shape and spread or irregularly with some long filopodia attached to the substrate. After 48 hours of culture, cells on all discs showed increased contact to each other and firm adhesion to the substrate with increased formation of filopodia compared to 24 hours of culture. Overall, the osteoblasts cultured on smooth zirconia group showed comparative initial adhesion properties and growth pattern, compared to the surface treated groups.
ALP assays were performed to compare the differentiation rate of osteoblasts on each group and the following results were obtained (Fig. 4). After 14 days of incubation, ALP was highest in the CaP group and then the ZS group with HA group the lowest. However, no significant differences were observed among the groups (P>.05).
XPS was used to determine the surface composition of the substrates of each group (Fig. 5). In all groups, the zirconia surface was oxidized as zirconium oxide and polluted by carbon contaminants in all groups. The CaP and HA samples also showed calcium and phosphorous peaks. However, the ZS samples exhibited zirconia, yttrium, and aluminum peaks. Samples with surface treatment showed peaks with calcium and phosphorous due to the different substances of the coating.
The cells were incubated for 24 h and the mRNA levels of type I collagen, osteocalcin, and osteonectin were analysed by RT-PCR. The levels of mRNA for type I collagen, osteocalcin, and osteonectin on the ZS, HA, and CaP groups were comparable and showed no significant differences (Fig. 6).
The dissolution rate of Ca2+ and P- was higher with CaP group than HA group (Fig. 7). Also, the Ca2+ and P- concentration increased with time for the CaP group. However, it decreased with time and became generally stable at 20 hours for the HA group.
Research for new bioactive coatings for dental implants to improve tissue integration and stability are still in progress.16,17 The topography and the surface chemistry are of great importance.18,19 Recently, surface-modified zirconia implants have been studied for long-term stability and strong bone tissue response.20 Monoclinic zirconia coated on titanium has been proved to have positive osteoblastic behavior and is potentially useful in hard tissue replacements.21 Calcium phosphate has been used as surface coatings on implants due to its bioactivity, which enables earlier stabilization of implants to the surrounding bone.22 Coatings with high dissolution behavior have high concentration of Ca2+ which enhances osteoblast responses and improves bone formation around the implant.23 However, the dissolution behavior and the low adhesion strength of the coating layer have raised concerns on the stability of the implants.24 In the present study, the concentration of Ca2+ and P- released from the coatings was higher with the CaP group than the HA group. Also, the Ca2+ and P- concentrations increased with time on the CaP group. However, they decreased or was stable with time on the HA group. The XPS results show that the ion compositions of the CaP and HA group are very similar. Thus, the different coating methods of the CaP or HA may explain the improved chemical stability of the HA group. This implies that aerosol deposition method can produce more stable coatings with implants and transgingival components. To verify the long-term stability of the zirconia coated with bioactive ceramics, evaluation of the adhesion strength between the coating and the substrate will be needed in the future study.
ALP activity is an important parameter that allows for the assessment of the differentiation level of the mineralization of osteoblasts, and is considered as a marker of the early stage of osteogenic differentiation.25 In this study, ALP activity of the CaP coated group was shown to be the highest. Although no significant differences were observed among the groups, it can be speculated that calcium phosphate coating may have positive effects on the early stage of osteogenic differentiation.
Type I collagens are major extracellular matrix components of osteoblasts and involved in adhesion. As osteocalcin is produced by osteoblasts, it is known as a marker for the bone formation process. Also, osteonectin is an extracellular matrix glycoprotein which is secreted by osteoblasts during bone mineralization and modulates cell proliferation. No significant differences were found in the level of mRNA for type I collagen, osteocalcin, and osteonectin during the 24 hours cell culture period.
Surface texture of machined zirconia is known to enhance bone apposition and has benefits on the removal torque values.6 However, in this study, smooth zirconia and surface coated zirconia showed overall comparable cellular viability which implies that surface chemistry affects osteoblast attachment and spreading. More in vivo and in vitro investigations are needed to establish the ideal surface roughness and biochemical coating for the zirconia implants.
From the semi-quantitative XPS analyses, it can be speculated that surface treatment affected the surface chemical composition of the zirconia surface. Although sandblasting with Al2O3 was not performed, XPS results documented the presence of Al on the smooth zirconia surface group which might have been incorporated for increasing the toughness of the zirconia.26 These Al2O3 particles would not affect the osseointegration pattern as shown by animal studies.27 However, the role of residual Al2O3 on implant surfaces is still a matter of controversy28 and it is difficult to conclude whether there is a positive or negative effect because of the low content of residual Al on the smooth zirconia surface group. Fluoride incorporation into the coating layer is known to bring about lower dissolution and greater chemical stability.29 Therefore, further studies on the incorporation of other ions and coating techniques for the best resistance to dissolution and higher positive cell stimulating effects are needed. Thus, we need to focus on the control of the in vivo degradation behavior and the mechanical properties of the coatings on the zirconia.
The attachment and growth behavior of bone marrowderived osteoblasts cultured on smooth zirconia and surface coated zirconia showed comparable results. However, considering the dissolution behavior of the surface coatings of the zirconia, the HA coating was more stable compared to the CaP coating. More in vitro and in vivo researches are necessary to identify a stable surface with controlled and standardized chemistry.
Figures and Tables
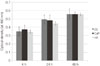 | Fig. 1Evaluation of cell proliferation using MTT assay for the ZS group, CaP group, and HA group. The data are expressed as the mean values (MV) ± standard deviation (SD) of three independent experiments. No significant differences were observed between the groups (P>.05). |
 | Fig. 2SEM observations of osteoblast cultures on surface-modified zirconia after 24 h incubation (×5,000). (A) ZS group at 24 h, (B) CaP group at 24 h, (C) HA group at 24 h. The cells were sporadically triangular or spread out in shape with long filopodia on all three groups of specimens. |
 | Fig. 3SEM observations of osteoblast cultures on surface-modified zirconia after 48 h incubation (×5,000). (A) ZS group at 48 h, (B) CaP group at 48 h, (C) HA group at 48 h. The cells showed more contact with each other and in intimate contact with the surface on all three groups of specimens. |
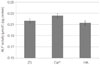 | Fig. 4Evaluation of the cellular differentiation by using ALP analysis after 14 days incubation. The ALP activity of osteoblasts on calcium phosphate coated zirconia was the highest. No significant differences were observed among the groups (P>.05). |
References
1. Pae A, Lee H, Kim HS, Kwon YD, Woo YH. Attachment and growth behaviour of human gingival fibroblasts on titanium and zirconia ceramic surfaces. Biomed Mater. 2009; 4:025005.
2. Mustafa K, Silva Lopez B, Hultenby K, Wennerberg A, Arvidson K. Attachment and proliferation of human oral fibroblasts to titanium surfaces blasted with TiO2 particles. A scanning electron microscopic and histomorphometric analysis. Clin Oral Implants Res. 1998; 9:195–207.
3. Pae A, Kim SS, Kim HS, Woo YH. Osteoblast-like cell attachment and proliferation on turned, blasted, and anodized titanium surfaces. Int J Oral Maxillofac Implants. 2011; 26:475–481.
4. Orsini G, Assenza B, Scarano A, Piattelli M, Piattelli A. Surface analysis of machined versus sandblasted and acid-etched titanium implants. Int J Oral Maxillofac Implants. 2000; 15:779–784.
5. Bächle M, Butz F, Hübner U, Bakalinis E, Kohal RJ. Behavior of CAL72 osteoblast-like cells cultured on zirconia ceramics with different surface topographies. Clin Oral Implants Res. 2007; 18:53–59.
6. Gahlert M, Gudehus T, Eichhorn S, Steinhauser E, Kniha H, Erhardt W. Biomechanical and histomorphometric comparison between zirconia implants with varying surface textures and a titanium implant in the maxilla of miniature pigs. Clin Oral Implants Res. 2007; 18:662–668.
7. Ichikawa Y, Akagawa Y, Nikai H, Tsuru H. Tissue compatibility and stability of a new zirconia ceramic in vivo. J Prosthet Dent. 1992; 68:322–326.
8. Josset Y, Oum'Hamed Z, Zarrinpour A, Lorenzato M, Adnet JJ, Laurent-Maquin D. In vitro reactions of human osteoblasts in culture with zirconia and alumina ceramics. J Biomed Mater Res. 1999; 47:481–493.
9. Scarano A, Piattelli M, Caputi S, Favero GA, Piattelli A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study. J Periodontol. 2004; 75:292–296.
10. Rimondini L, Cerroni L, Carrassi A, Torricelli P. Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int J Oral Maxillofac Implants. 2002; 17:793–798.
11. Degidi M, Artese L, Scarano A, Perrotti V, Gehrke P, Piattelli A. Inflammatory infiltrate, microvessel density, nitric oxide synthase expression, vascular endothelial growth factor expression, and proliferative activity in peri-implant soft tissues around titanium and zirconium oxide healing caps. J Periodontol. 2006; 77:73–80.
12. Ismail FS, Rohanizadeh R, Atwa S, Mason RS, Ruys AJ, Martin PJ, Bendavid A. The influence of surface chemistry and topography on the contact guidance of MG63 osteoblast cells. J Mater Sci Mater Med. 2007; 18:705–714.
13. Mustafa K, Wennerberg A, Arvidson K, Messelt EB, Haag P, Karlsson S. Influence of modifying and veneering the surface of ceramic abutments on cellular attachment and proliferation. Clin Oral Implants Res. 2008; 19:1178–1187.
14. Hulbert SF, Young FA, Mathews RS, Klawitter JJ, Talbert CD, Stelling FH. Potential of ceramic materials as permanently implantable skeletal prostheses. J Biomed Mater Res. 1970; 4:433–456.
15. Maniatopoulos C, Sodek J, Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988; 254:317–330.
16. Kim HW, Kong YM, Bae CJ, Noh YJ, Kim HE. Sol-gel derived fluor-hydroxyapatite biocoatings on zirconia substrate. Biomaterials. 2004; 25:2919–2926.
17. Takemoto M, Fujibayashi S, Neo M, Suzuki J, Kokubo T, Nakamura T. Bone-bonding ability of a hydroxyapatite coated zirconia-alumina nanocomposite with a microporous surface. J Biomed Mater Res A. 2006; 78:693–701.
18. Boyan BD, Batzer R, Kieswetter K, Liu Y, Cochran DL, Szmuckler-Moncler S, Dean DD, Schwartz Z. Titanium surface roughness alters responsiveness of MG63 osteoblast-like cells to 1 alpha,25-(OH)2D3. J Biomed Mater Res. 1998; 39:77–85.
19. Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Relat Res. 1981; 157:259–278.
20. Sennerby L, Dasmah A, Larsson B, Iverhed M. Bone tissue responses to surface-modified zirconia implants: A histomorphometric and removal torque study in the rabbit. Clin Implant Dent Relat Res. 2005; 7:S13–S20.
21. Wang G, Meng F, Ding C, Chu PK, Liu X. Microstructure, bioactivity and osteoblast behavior of monoclinic zirconia coating with nanostructured surface. Acta Biomater. 2010; 6:990–1000.
22. Sun L, Berndt CC, Gross KA, Kucuk A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: a review. J Biomed Mater Res. 2001; 58:570–592.
23. Zeng H, Lacefield WR. The study of surface transformation of pulsed laser deposited hydroxyapatite coatings. J Biomed Mater Res. 2000; 50:239–247.
24. de Groot K, de Putter C, Smitt P, Driessen A. Mechanical failure of artificial teeth made of dense calcium hydroxyapatite. Sci Ceram. 1981; 11:433–437.
25. Cui FZ, Luo ZS, Feng QL. Highly adhesive hydroxyapatite coatings on titanium alloy formed by ion beam assisted deposition. J Mater Sci Mater Med. 1997; 8:403–405.
26. Benzaid R, Chevalier J, Saâdaoui M, Fantozzi G, Nawa M, Diaz LA, Torrecillas R. Fracture toughness, strength and slow crack growth in a ceria stabilized zirconia-alumina nanocomposite for medical applications. Biomaterials. 2008; 29:3636–3641.
27. Piattelli A, Degidi M, Paolantonio M, Mangano C, Scarano A. Residual aluminum oxide on the surface of titanium implants has no effect on osseointegration. Biomaterials. 2003; 24:4081–4089.
28. Zinelis S, Thomas A, Syres K, Silikas N, Eliades G. Surface characterization of zirconia dental implants. Dent Mater. 2010; 26:295–305.
29. Jha LJ, Best SM, Knowles JC, Rehman I, Santos JD, Bonfield W. Preparation and characterization of fluoride-substituted apatites. J Mater Sci Mater Med. 1997; 8:185–191.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download